Membrane Potentials
Electrochemical potentials are
present across the membranes of virtually all cells in the body. Some cells, such as nerve and muscle cells, are
capable of generating rapidly changing electrical impulses, and these impulses
are used to transmit signals along their membranes. In other cells, such as
glandular cells, membrane potentials are used to signal the release of hormones
or activate other functions of the cell. Generation of membrane potentials
relies on (1) diffusion of current-carrying ions, (2) development of an electrochemical
equilibrium, (3) establishment of a RMP, and (4) triggering of action potentials.
Diffusion Of Current-Carrying
Ions
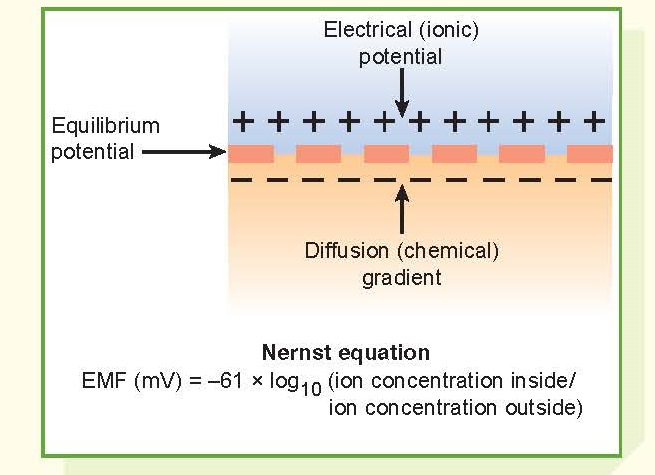
A diffusion potential is a
potential difference generated across a membrane when a current-carrying ion,
such as the potassium (K+) ion, diffuses down its concentration
gradient. Two conditions are necessary for this to occur: (1) the membrane must
be selectively permeable to a particular ion, and (2) the concentration of the
diffusible ion must be greater on one side of the membrane than the other.
The magnitude of the diffusion
potential, measured in millivolts, depends on the size of the concentration
gradient. The sign (+ or −) or polarity of the potential depends on the
diffusing ion. It is negative on the inside when a positively charged ion such
as K+ diffuses from the inside
to the outside of the membrane, carrying its charge with it.
Equilibrium Potentials
An equilibrium potential is the
membrane potential that exactly balances and opposes the net diffusion of an
ion down its concentration gradient. As a cation diffuses down its
concentration gradient, it carries its positive charge across the membrane,
thereby generating an electrical force that will eventually retard and stop its
diffusion. An electrochemical equilibrium is one in which the chemical
forces driving diffusion and the repelling electrical forces are
exactly balanced so that no further diffusion occurs. The equilibrium potential
(EMF, electromotive force) can be calculated by inserting the inside and
outside ion concentrations into
the Nernst equation.
Resting Membrane Potential
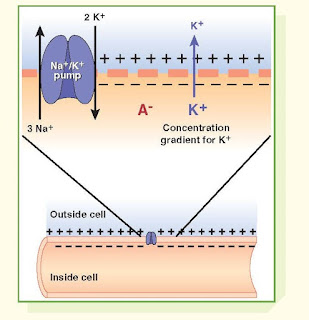
The RMP, which is necessary for
electrical excitability, is present when the cell is not transmitting impulses. Because the resting membrane is
permeable to K+, it is essentially a K+ equilibrium
potential. This can be explained in terms of the large K+
concentration gradient (e.g., 140 mEq/L inside and 4 mEq/L outside),
which causes the positively charged K+ to diffuse outward, leaving
the nondiffusible, negatively charged intracellular anions (A−)
behind. This causes the membrane to become polarized, with negative charges
aligned along the inside and positive charges along the outside. The Na+/K+
membrane pump, which removes three Na+ from inside while returning only two K+
to the inside, contributes to the
maintenance of the RMP.
Action Potentials
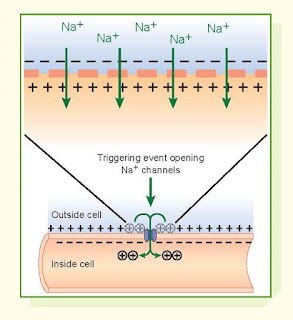
Action potentials involve rapid
changes in the membrane potential. Each
action potential begins with a sudden change from the negative RMP to a
positive threshold potential, causing an opening of the mem- brane channels for
Na+ (or other ions of the action potential). Opening of the Na+
channels allows large amounts of the positively charged Na+ ions to diffuse to
the interior of the cell, causing the membrane potential to undergo
depolarization or a rapid change to positive on the inside and negative on the
outside. This is quickly followed by closing of Na+ channels and
opening of the K+ channels, which leads to a rapid efflux of K+ from the cell and
reestablishment of the RMP.