Sunday, May 11, 2025
Friday, April 25, 2025
TRAUMA TO PENIS AND URETHRA
Sunday, April 20, 2025
CYSTS AND CANCER OF THE SCROTUM
Tuesday, November 19, 2024
Delayed Or Absent Puberty
Saturday, September 30, 2023
INTERSEX FEMALE PSEUDOHERMAPHRODITISM
Sunday, March 26, 2023
Tuesday, March 21, 2023
Lactation
Monday, March 20, 2023
Prenatal Screening and Diagnosis
Wednesday, February 15, 2023
Fertilization And The Establishment Of Pregnancy
Tuesday, February 14, 2023
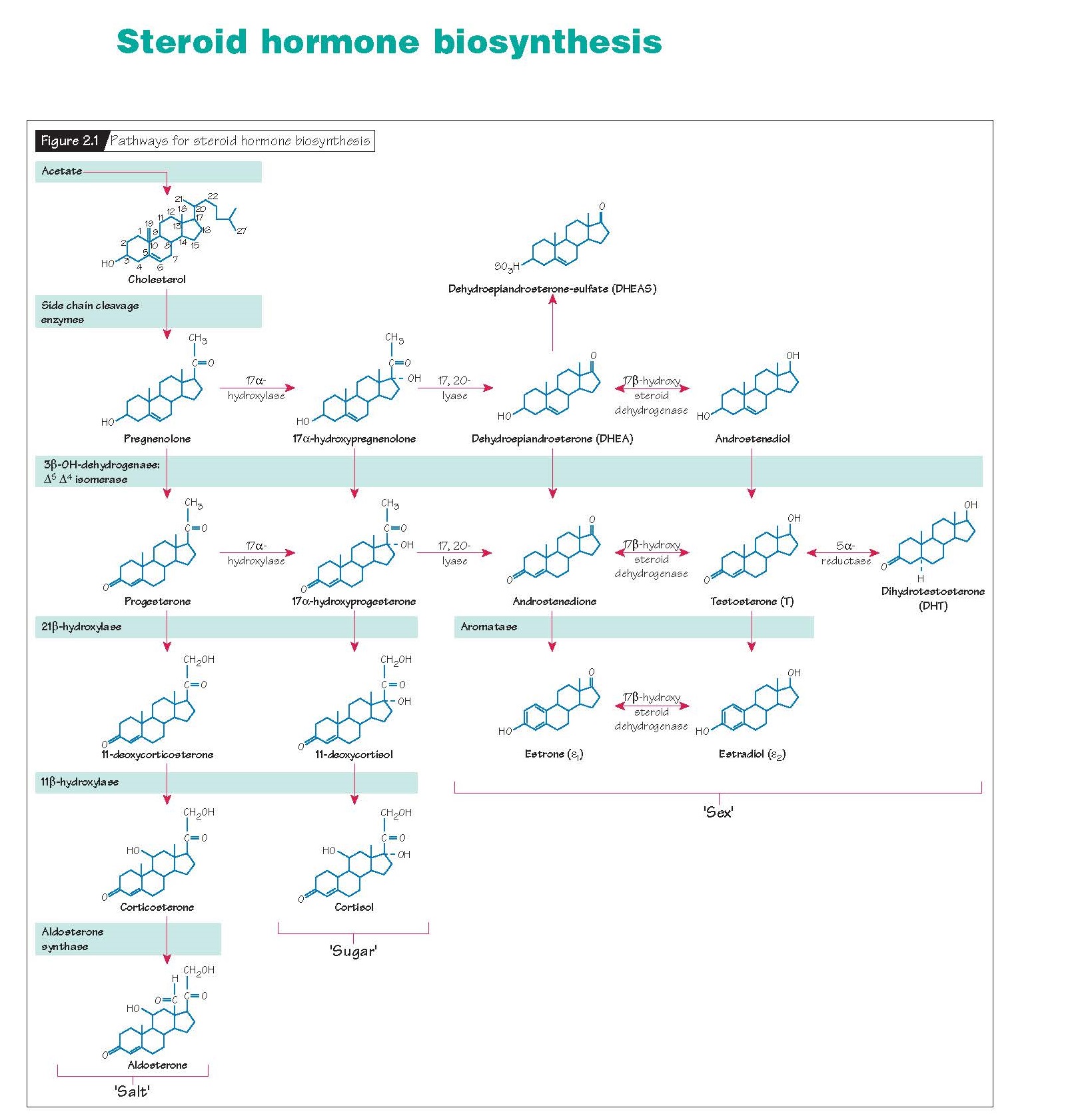
Steroid Hormone Biosynthesis
Thursday, February 2, 2023
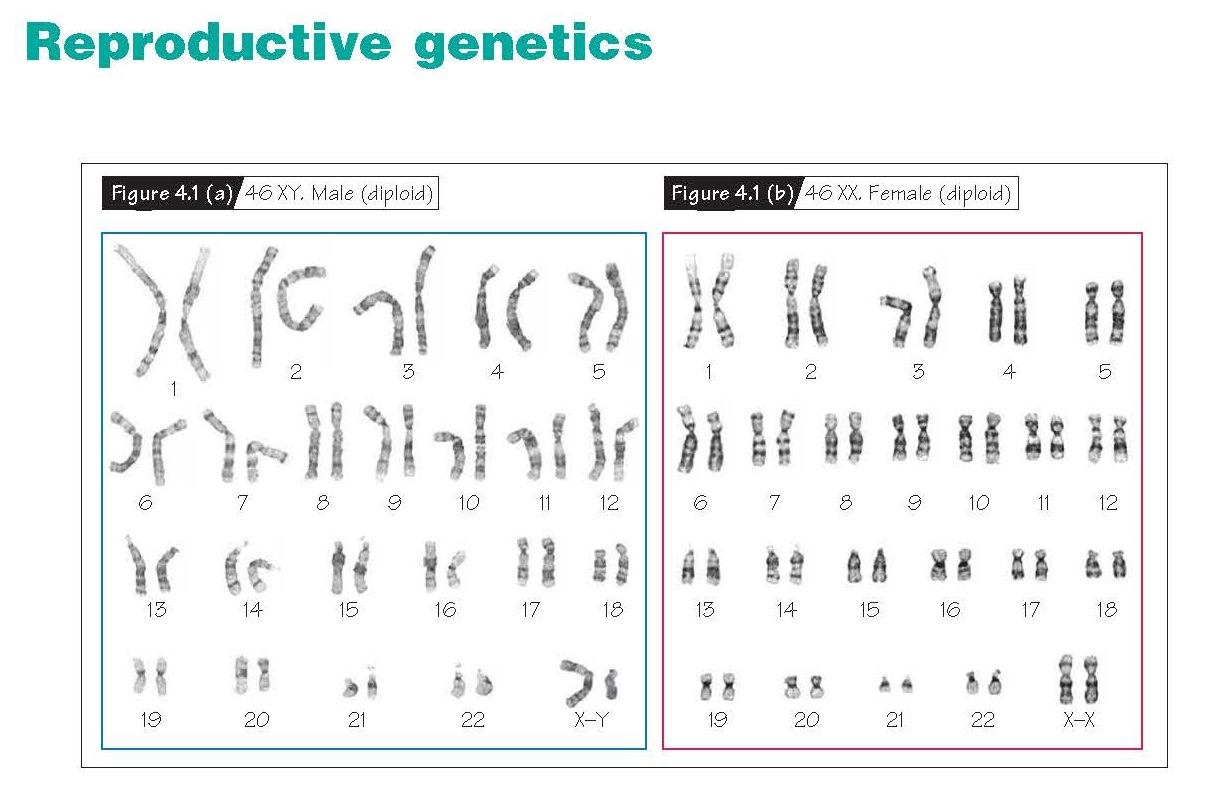
Reproductive Genetics
Tuesday, January 31, 2023
Musculoskeletal System: Limbs
Skeletal System
Wednesday, December 1, 2021
PUERPERAL INFECTION
PUERPERAL
INFECTION
Puerperal infection generally refers to an infection of the genital
tract in the postpartum period. For centuries, puerperal infection was the
leading cause of maternal death, though this has changed dramatically with the
advent of antibiotics. Maternal death rates associated with infection account
for approximately 0.6 maternal deaths per 100,000 live births. Endometritis is
the most common form of postpartum infection, though other sources of
postpartum infections include postsurgical wound infections, perineal
cellulitis, mastitis, respiratory complications from anesthesia or under-lying
pulmonary disease such as asthma or obstructive lung disease, retained products
of conception, urinary tract infections, and septic pelvic phlebitis. Overall,
postpartum infection is estimated to affect 1% to 3% of normal vaginal
deliveries, 5% to 15% of scheduled caesarean deliveries, and 15% to 20% of
unscheduled caesarean deliveries.
The organisms responsible for the vast majority of puerperal infections are the anaerobic and aerobic nonhemolytic varieties of streptococci. These organisms are usually present in the birth canal, becoming pathogenic when carried to the uterine cavity during or after delivery.
ERYTHROBLASTOSIS FETALIS (RH SENSITIZATION)
ERYTHROBLASTOSIS
FETALIS (RH SENSITIZATION)
Isoimmunization of the mother to any dissimilar
fetal blood group not possessed by the mother is possible. Historically the
most common example is the Rh (D) factor. Erythroblastosis fetalis (hemolytic
disease of the newborn) is characterized by sustained destruction of the fetal
erythrocytes by specific maternal antibodies (IgG), which cross the placenta to
the fetus. What was once a common cause for fetal death has largely been
eradicated by prophylactic maternal administration of immune globulin against
the Rh (D) factor to those at risk.
Human red blood cells contain a complex group of inherited antigens, one of which is the Rhesus CDE antigen system. The genes for the CDE blood groups are inherited separately from the ABO groups and are located on the short arm of chromosome 1. One of the more important antigens of this group is Rh (D) factor. About 85% of all individuals are Rh (D)-positive whereas 15% are Rh (D)-negative. Any process that exposes the woman to blood carrying the D antigen including blood transfusion, miscarriage, ectopic or normal pregnancy, trauma during pregnancy, amnio-centesis, and others can result in anti-Rh agglutinins being formed. The IgG antibodies can cross the placenta into the fetal circulation and result in the destruction of the Rh-positive fetal blood. Other isoimmunizations (most frequently Kell, or Duffy antigens) can also result in similar effects on the fetus.
SYPHILIS
SYPHILIS
In
many geographical regions, syphilis is still the most common cause of fetal
death in the later months of gestation. In many developed countries, the number
of primary and secondary syphilis cases rose dramatically during the late 1980s
and early 1990s (peak 1991) as a result of illicit drug use and the exchange of
drugs for sex. Although rare in developed countries, the incidence of syphilis
is high and increasing in many developing countries (and in the transitional
economies of Eastern Europe and the former Soviet Union), particularly where
HIV/AIDS is common. Of infants born to mothers with primary or secondary
syphilis, up to 50% will be premature, stillborn, or die in the neonatal
period. In many cases, surviving children are born with congenital defects some
of which may not be apparent for years.
The fetus is infected through the placenta from the mother. When an infected fetus is born alive, the symptomatology of congenital syphilis soon becomes manifest. While appropriate treatment of the mother can prevent congenital syphilis, the inability to effectively identify infected patients and get them to undergo treatment continues to present a challenge to reducing the incidence of syphilitic complications. Screening in the first trimester with nontreponemal tests such as rapid plasma reagin or Venereal Disease Research Laboratory test combined with confirmation of reactive individuals with treponemal tests such as the fluorescent treponemal antibody absorption (FTA-ABS) assay is a cost-effective strategy. Those at risk should be retested in the third trimester.
INTRAUTERINE GROWTH RESTRICTION
INTRAUTERINE
GROWTH RESTRICTION
Symmetric or asymmetric reduction in the size
and weight of the growing fetus in utero, compared with that expected for a
fetus of comparable gestational age, constitutes intrauterine growth
restriction. This reduced growth may occur for many reasons, but most
occurrences represent signs of significant risk of fetal death or jeopardy to
the fetus. Some authors advocate identifying fetuses with growth between the
10th and 20th percentiles as suffering “diminished” growth and at intermediate
risk for complications. Problems of consistent definition make estimates of the
true prevalence of growth restriction difficult, but by most definitions it
occurs in 5% to 10% of pregnancies.
The risk of intrauterine growth restriction increases with the presence of maternal conditions that reduce placental perfusion (hypertension, preeclampsia, drug use, smoking) or those that reduce the nutrients available to the fetus (chronic renal disease, poor nutrition, inflammatory bowel disease). Abnormalities of placental implantation or function can result in significant reduction in nutrient flow to the fetus. The risk is also higher at the extremes of maternal age: for women less than 15 years old the rate of low birth weight is 13.6% compared with 7.3% for women between 25 and 29 years old. When multiple gestations are excluded, the rate for women older than 45 years is greater than 20%. Multiple pregnancies, especially higher order multiples, are at increased risk for growth restrictions. In most cases of growth restriction, no specific cause is identified.
CAUSES OF DECREASED MATERNAL CIRCULATION
CAUSES
OF DECREASED MATERNAL CIRCULATION
Various pathologic conditions may impede the
maternal circulation to the placenta. They can be grouped as follows:
1. Diseases
of the uterine vessels: (a) acute atherosis, (b) arteriolar sclerosis associated with
essential hypertension, and (c) inflammation (angiitis) associated with
chorioamnionitis.
2. Premature
separation of the placenta associated with retroplacental hemorrhage or
inflammatory exudation.
3. Conditions
that may cause an increase in intra-uterine pressure: (a) multiple pregnancy,
(b) macrosomia, (c) polyhydramnios, and (d) hydatidiform mole.
4.
Extensive
thrombosis of the intervillous space or of the marginal sinus.
5.
Death
of the mother.
The most common cause of placental infarcts in cases of preeclampsia have been found to be acute atherosis of the decidual vessels. This lesion is manifested microscopically as a deposition of lipids, in the intima of decidual arterioles and endometrial arteriovenous lakes. Part of the material is doubly refractive under polarized light and occurs both extracellularly and inside lipophages. The lesions closely resemble acute fulminating atherosis in other settings. The process leads to marked intimal thickening and vascular occlusion. The lesions occur in the decidua vera, as well as in the basalis, but they do not involve to a comparable degree the vessels of the myometrium or other tissues in the body. Fat stains have not revealed the lesion in fetal vessels. Contiguous trophoblastic tissue seems to be a necessary factor in its pathogenesis. The lesions regress promptly after delivery. The cause of this condition is still unknown.
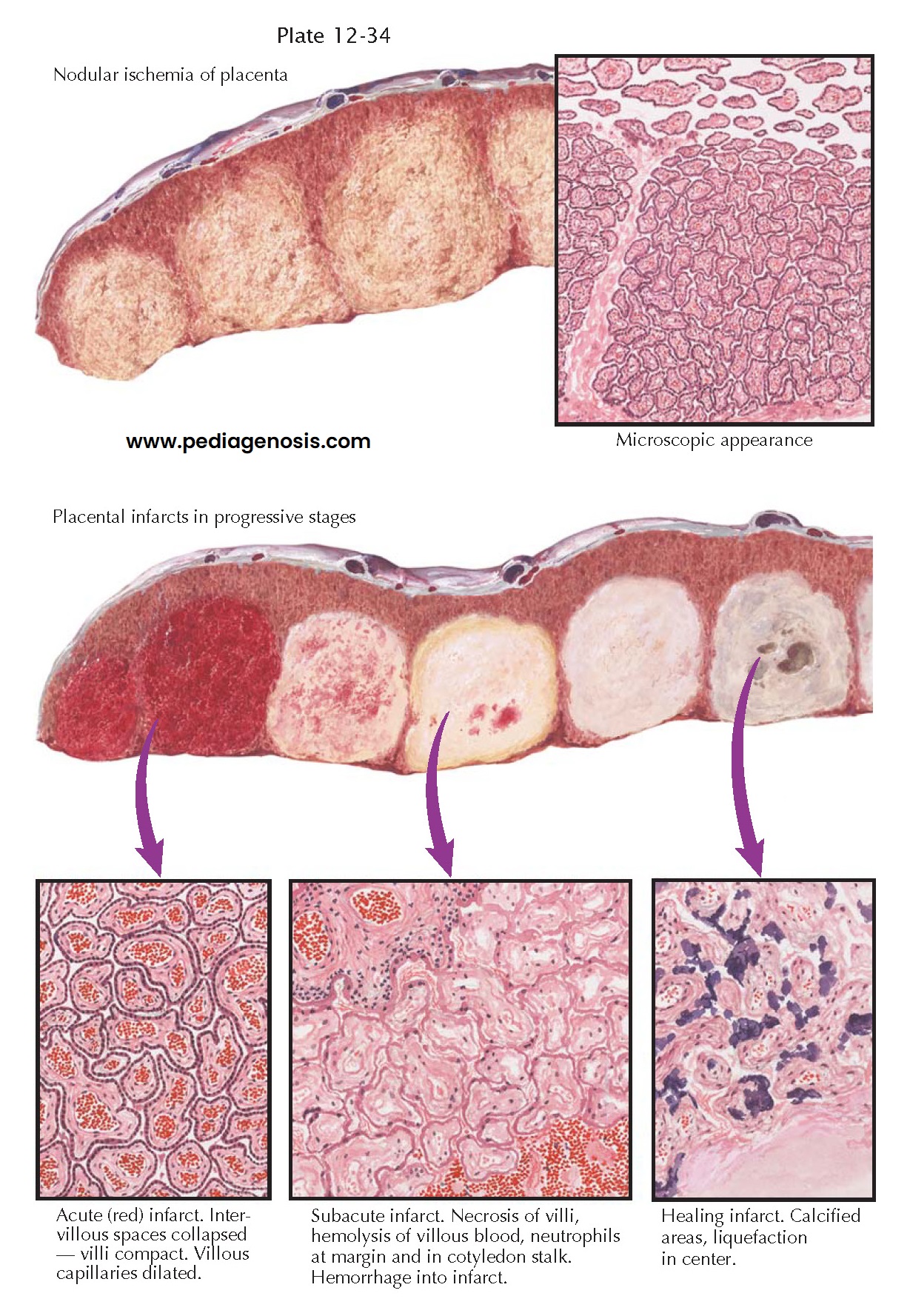
PREECLAMPSIA IV-PLACENTAL INFARCTS
PREECLAMPSIA
IV-PLACENTAL
INFARCTS
Because preeclampsia is a
condition peculiar to pregnancy, and because delivery usually results in the
rapid regression of the signs, symptoms, and pathologic lesions, it would seem
reasonable to believe that the contents of the gravid uterus either may be the
source of the vasoconstrictor factor or may play an important role in leading
to the production of that factor elsewhere in the body. The fetus is not a
required factor, because severe preeclampsia occasionally accompanies
hydatidiform mole. Therefore, the trophoblastic tissue or the gravid
endometrium would seem to be incriminated. Despite this, there are no micro- or
macroscopic placental lesions that are pathognomonic for preeclampsia.
Pathologic studies have revealed close correlation between the occurrence of preeclampsia and conditions that are prone to cause a decrease in the maternal circulation to the placenta, to the decidua, or to both of these tissues. Obstruction of the maternal blood flow to one or more placental cotyledons causes true infarction of the involved areas. Unfortunately, the term infarct has often been used for a wide variety of nodular lesions in the placenta, and conflicting opinions have been expressed concerning the association of such lesions with preeclampsia.
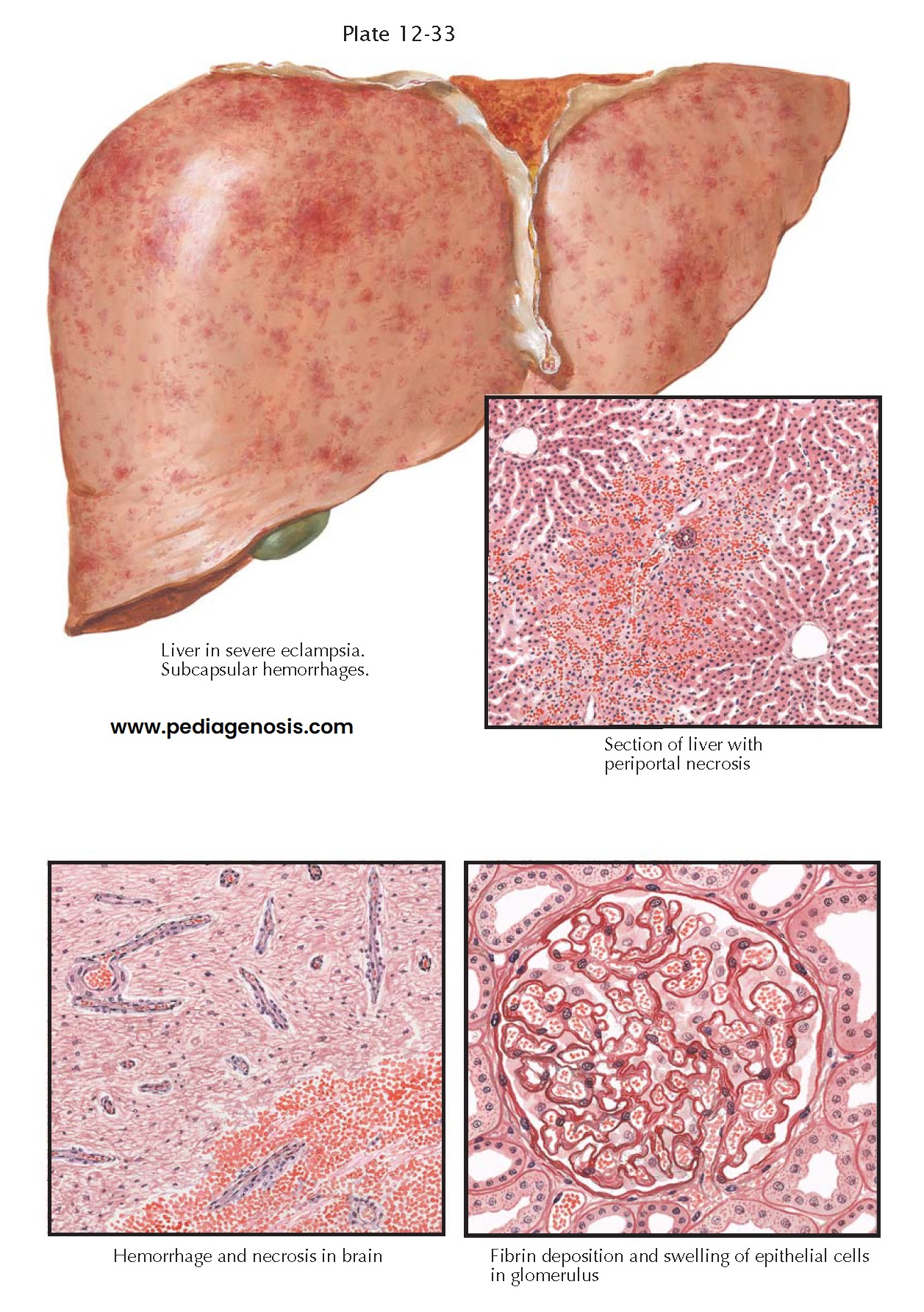
PREECLAMPSIA III—VISCERAL LESIONS IN PREECLAMPSIA AND ECLAMPSIA
PREECLAMPSIA
III—VISCERAL LESIONS IN PREECLAMPSIA AND ECLAMPSIA
Although preeclampsia and eclampsia are
differentiated, depending upon whether or not the patient has had a convulsion,
the pathology of the two is essentially the same. Characteristic lesions
frequently appear in the liver, kidneys, and brain, but they are inconstant in
occurrence and may be absent even in severe cases with convulsions. Therefore,
they cannot be considered primary lesions but are probably the sequelae of the
three constantly present features of the disease, namely, vasoconstriction,
hypertension, and fluid retention.
In typical cases, the liver is swollen and mottled with small hemorrhages. Microscopically, the sinusoids around the smaller portal areas are plugged with fibrinoid material and surrounded by foci of hemorrhage and necrotic liver cells. Occasionally, midzonal necrosis is seen, but serial sections usually reveal continuity with larger periportal lesions. The condition may be wide- spread or may involve only a few subcapsular lobules.