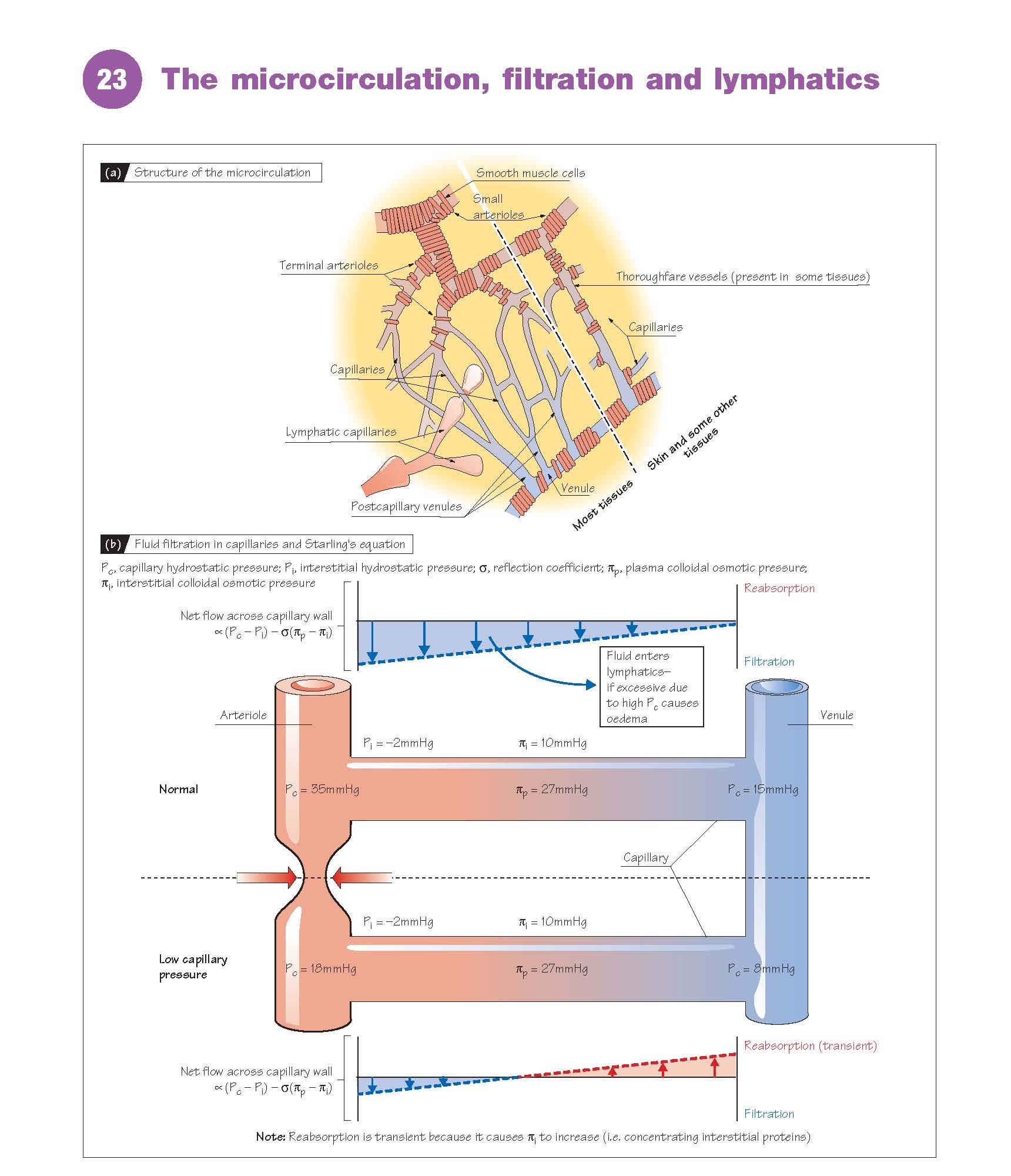
The
microcirculation is perhaps the raison d’être for the
cardiovascular system, as it is here that exchange between blood and tissues
occurs. It consists of the smallest (terminal) arterioles and the
exchange vessels capillaries and small venules (Chapter
16). Blood flow into the microcirculation is regulated by the vasoconstriction
of small arterioles, activated by sympathetic stimulation through numerous
nerve endings in their walls (Chapters 7 and 22). Each small arteriole feeds
many capillaries via several terminal arterioles (Fig. 23a), which are
not innervated. Instead, the vasoconstriction of terminal arterioles is
mediated by local metabolic products (Chapter 24), allowing perfusion to
be matched to metabolism. A few tissues (e.g. mesenteric, skin) have thoroughfare
vessels connecting small arterioles and venules directly. Note that the
term ‘pre-capillary sphincter’ is misleading and should be avoided, as
no such anatomical structures exist.
Transcapillary exchange
Water, gases and other substances
cross the capillary wall mainly by diffusion down their concentration
gradients (Chapter 11). O2 and CO2 are highly lipophilic (soluble in
lipids), and can cross the endothelial lipid bilayer membrane easily. This is,
however, impermeable to hydrophilic (‘waterloving’, lipidinsoluble)
molecules, such as glucose, and polar (charged) molecules and ions
(electrolytes). Such substances mainly cross the wall of continuous
capillaries through the gaps between endothelial cells. This is slowed by tight
junctions between cells and by the glycocalyx (Chapter 21), so that
diffusion is 1000–10 000 times slower than for lipophilic substances. This small
pore system also prevents the diffusion of substances greater than 10 000
Da (e.g. plasma proteins). The latter can cross the capillary wall, but
extremely slowly; this may involve large pores through endothelial
cells. Fenestrated capillaries (gut, joints, kidneys) are 10fold more
permeable than continuous capillaries because of pores called fenestrae (from
the Latin for ‘windows’), whereas discontinuous capillaries are
highly permeable due to large spaces between endothelial cells, and occur where
red cells need to cross the capillary wall (bone marrow, spleen, liver)
(Chapter 21).
Filtration (Fig. 23b)
The capillary walls are much more
permeable to water and electrolytes than to proteins (see above). The
concentration of electrolytes (e.g. Na+, Cl−), and therefore the osmotic
pressure exerted by them (crystal- loid osmotic pressure), is very
similar in plasma and interstitial fluid, and has little effect on fluid
movement. The protein concentration in plasma however is greater than that in
interstitial fluid, and the component of osmotic pressure exerted by proteins (colloidal
osmotic or oncotic pressure)
in the plasma (∼27 mmHg) is therefore greater than in the interstitial fluid (∼10
mmHg). Water tends to flow from a low to a high osmotic
pressure, but from a high to a low hydrostatic pressure. The net flow of water across the
capillary wall is therefore determined by the balance between the hydrostatic
(P) and colloidal osmotic (π) pressures, according to Starling’s
equation, flow ∝ (Pc − Pi) − σ(πp −
πi), where (Pc − Pi) is the difference in hydrostatic pressure between
capillary and interstitial fluid, and (πp − πi) is the difference in colloidal
osmotic pressure between plasma and interstitial fluid; (πp − πi) has an
average value of ∼17 mmHg. σ is the reflection coefficient (∼0.9), a
measure of how difficult it is for plasma proteins to cross the capillary wall.
Note that the interstitial protein concentration, and therefore πi, differs
between tissues; in the lung for example (πp − πi) is ∼13 mmHg.
The capillary hydrostatic pressure
normally varies from ∼35 mmHg at the arteriolar end to ∼15 mmHg
at the venous end, whereas the interstitial hydrostatic pressure is
approximately –2 mmHg. (Pc − Pi) is therefore greater than σ(πp − πi) along the
length of the capillary, resulting in the net filtration of water into
the interstitial space (Fig. 23b). Although arteriolar constriction will reduce
capillary pressure and therefore lead to the reabsorption of fluid, this will
normally be transient due to the concentration of interstitial fluid (i.e.
increased πi). A reduction in plasma protein (e.g. starvation), or a
loss of endothelium integrity and thus diffusion of protein into the
interstitial space (e.g. severe
inflammation, ischaemia), will similarly reduce (πp − πi), leading
to enhanced filtration and loss of fluid into the tissues. This is also caused
by a high venous pressure (oedema; see below).
Lymphatics
Fluid filtered by the
microcirculation (∼8 L per day) is returned to the blood by the lymphatic system. Lymphatic
capillaries are blindended bulbous
tubes (diameter, ∼15–75 μm) walled with endothelial cells (Fig. 23a).
These allow the entry of fluid, proteins
and bacteria, but prevent their
exit. Lymphatic capillaries merge into collecting lymphatics and then
larger lymphatic vessels, both containing smooth muscle and unidirectional
valves. Lymph is propelled, by smooth muscle constriction and compression
of the vessels by body movement, into afferent lymphatics and then the lymphatic
nodes, where bacteria and other foreign materials are removed by
phagocytes.
Most fluid is reabsorbed here by capillaries,
with the remainder returning via efferent lymphatics and the thoracic
duct into the subclavian veins. Lymphatics are also important for lipid
absorption in the gut.
Oedema
Oedema is swelling of the tissues
due to excess fluid in the interstitial space. It is caused when filtration is
increased to the extent that the lymphatics are unable to remove the fluid fast
enough (see above), or by dysfunctional lymphatic drainage (e.g. elephantiasis,
the blockage of lymphatics with filarial nematode worms). Inflammation (Chapter
10) causes swelling and oedema because it
increases capillary permeability, allowing protein to leak into the
interstitium and disrupt the oncotic pressure gradient, so filtration is
increased. Reduced venous drainage (increased venous pressure) also increases
filtration and can lead to oedema; standing without moving the legs prevents
the opera tion of the muscle pump (Chapter 16), local venous pressure
rises, and the legs swell. In congestive heart failure, reduced cardiac
function results in increased pulmonary and central venous pressure (Chapter
20), leading, respectively, to pulmonary oedema (alveoli fill with
fluid) and peripheral oedema [swelling of the legs and liver, and
accumulation of fluid in the peritoneum (ascites)]. Severe protein
starvation can cause
generalized oedema and
a grossly swollen abdomen due to ascites and an enlarged liver (kwashiorkor).