Immunity To Bacteria
Unlike viruses, bacteria are cellular organisms, mostly capable of fully
independent life, but some live on or in
larger animals some or all of the time. Indeed, it is estimated that each human
is colonized by some 1014 bacteria, equivalent to 10 bacteria for every cell of
the body. This microbiome is made up of several thousand different
species, most of which are innocuous and may even have a beneficial role in
enhancing human health. However, a few species can cause disease and, together
with viruses, these now constitute the major infectious threat to health in
developed countries. Since the discovery of antibiotics, bacterial infection
has been controlled largely by chemotherapy. However, with the recent rise in
antibiotic-resistant strains of bacteria, there is renewed interest in
developing new or improved vaccines against the bacteria responsible for such
diseases as tuberculosis, meningitis and food poisoning.
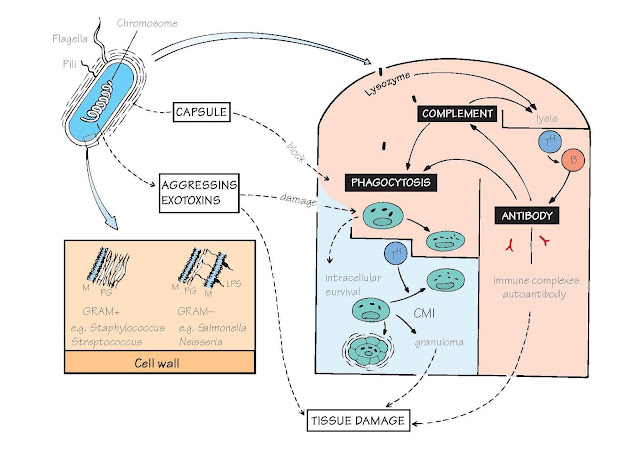
The usual destiny of unsuccessful
bacteria is death by phagocytosis; survival therefore entails avoidance of this fate. The main ways in
which a bacterium (top left) can achieve this lie in the capsule (affecting
attachment), the cell wall (affecting digestion) and the release of exotoxins
(which damage phagocytic and other cells). Fortunately, most capsules and
toxins are strongly antigenic and antibody overcomes many of their effects;
this is the basis of the majority of antibacterial vaccines. In the figure,
processes beneficial to the bacteria or harmful to the host are shown in broken
lines. Bacteria living on body surfaces (e.g. teeth) can form colonies
(‘biofilms’) which protect them against both immunity and antibiotics. As with
viruses, some of the most virulent and obstinate bacterial infections are zoonoses
– plague (rats) and brucellosis (cattle) being examples. Bacteria that manage
to survive in macrophages (e.g. tuberculosis [TB]) can induce severe
immune-mediated tissue damage (see Fig. 37).
Cell wall Outside their plasma membrane (M in the figure)
bacteria have a cell wall composed
of a mucopeptide called peptidoglycan (PG); it is here that lysozyme acts
by attacking the N-acetylmuramic acid–N-acetylglucosamine links.
In addition, Gram-negative bacteria have a second membrane with
lipopolysaccharides (LPS, also called endotoxin) inserted in it. Bacterial cell
walls are powerful inducers of inflammation, largely through their ability to
activate the Toll-like receptors of innate immunity (see Figs 3 and 5).
Flagella, the main agent of bacterial motility, contain
highly antigenic proteins (the ‘H antigens’ of typhoid, etc.), which give rise
to immobilizing antibody. Some flagellar proteins activate the Toll-like receptor
TLR5 (see Fig. 5).
Pili are used by bacteria to adhere to cells; antibody can prevent this
(e.g. IgA against gonococcus).
Capsule Many bacteria owe their virulence to capsules,
which protect them from contact with phagocytes. Most are large, branched, polysaccharide
molecules, but some are protein. Many of these capsular polysaccharides, and
also some proteins from flagella, are T-independent antigens (see Fig. 19).
Examples of capsulated bacteria are pneumococcus, meningococcus and Haemophilus
spp.
Exotoxins (as distinct from the endotoxin [LPS] of cell
walls) Gram-positive bacteria often secrete proteins with destructive effects
on phagocytes, local tissues, the CNS, etc.; frequently, these are the cause of
death. In addition there are proteins collectively known as aggressins that
help the bacteria to spread by dissolving host tissue.
Sepsis Occasionally, uncontrolled systemic responses
to bacterial infection develop, which can lead to rapid life-threatening
disease (‘toxic shock’). Such responses are still an important cause of death
after major surgery. Over-production of TNF-α, especially by macro- phages, has
a major role in these reactions.
Here, bacteria are given their
popular rather than their proper taxonomic names. Some individual aspects of
interest are listed below:
Strep Streptococcus, classified either by haemolytic exotoxins (α,
β, γ) or cell wall antigens (groups A–Q). Group A β-haemolytic are the most
pathogenic, possessing capsules (M protein) that attach to mucous membranes but
that resist phagocytosis, numerous exotoxins (whence scarlet fever),
indigestible cell walls causing severe cell-mediated reactions, antigens that
cross-react with cardiac muscle (rheumatic fever) and a tendency to
kidney-damaging immune complexes.
Staph Staphylococcus. Antiphagocytic factors include the fibrinforming
enzyme coagulase and protein A, which binds to the Fc portion of IgG, blocking
opsonization. Numerous other toxins make staphylococci highly destructive,
abscess-forming organisms. Large-scale use of antibiotics has caused the
emergence of bacterial strains resistant to many antibiotics
(methicillin-resistant Staphyloccus aureus [MRSA]), which are now
proving a serious threat, particularly as hospital-acquired infections.
Pneumococcus (now S. pneumoniae), meningococcus Typed
by the polysaccharides of their capsules, and especially virulent in the
tropics, where vaccines made from capsular polysaccharides are proving highly
effective in preventing epidemics. Also more common in patients with deficient antibody responses
(see Fig. 33). Chemical coupling of
the capsular polysaccharides to a protein, such as diphtheria toxoid, converts
these antigens from T-cell independent to T-cell dependent, thus greatly
increasing memory and potency. Such conjugate vaccines have proven highly
effective at preventing childhood meningitis and Haemophilus infection.
Gonococcus IgA may block attachment to mucous surfaces,
but the bacteria secrete a protease that destroys the IgA; thus, the infection
is seldom eliminated, leading to a ‘carrier’ state. Gonococci and meningococci
are the only bacteria definitely shown to be disposed of by complement-mediated
lysis.
Tuberculosis and leprosy bacilli
These mycobacteria have very
tough cell walls, rich in lipids, which resist intracellular killing; they can
also inhibit phagosome–lysosome fusion. Chronic cell-mediated immunity results
in the formation of granuloma, tissue destruction and scarring (see Fig. 37).
In leprosy, a ‘spectrum’ between localization and dissemination corresponds to
the predominance of cell-mediated immunity and of antibody, respectively.
Tuberculosis is once again on the rise, partly as a result of increased travel,
partly because of increased drug resistance and partly as a consequence of
AIDS, and better vaccines to replace the only partially effective BCG (bacille
Calmette– Guérin) are urgently being sought.
Escherichia coli is now perhaps the best-known bacterial species
in the world, because of its ubiquitous use as a tool in all molecular biology
laboratories. However, the species is a made up of an enormous number of
different strains. Most are harmless inhabitants of the intestine of many
mammals including humans, and may even be beneficial in sup- plying some
vitamins and in suppressing the growth of other pathogenic bacteria. But a few
strains produce exotoxins and have been responsible for major outbreaks of food
poisoning. Shigella (causing dysentery) and cholera are
two other examples of bacteria that grow only in the intestine, and are
responsible for important human diseases.
Salmonella (e.g. S. typhi) also infects the
intestine but can survive and spread to other parts of the body within
macrophages. Recovery after infections may lead to a ‘carrier’ state.
Tetanus owes its severity to the rapid action of its
exotoxin on the CNS. Antibody (‘antitoxin’) is highly effective at blocking
toxin action, an example where neither complement nor phagocytic cells are
needed.
Diphtheria also secretes powerful neurotoxins, but death
can be due to local tissue damage in the larynx (‘false membrane’).
Syphilis is an example of bacteria surviving all forms
of immune attack without sheltering inside cells. The commonly found autoantibody
to mitochondrial cardiolipin is the basis of the diagnostic Wasserman reaction.
Cross-reactions of this type, due presumably to bacterial attempts to mimic
host antigens and thus escape the attentions of the immune system, are clearly
a problem to the host, which has to choose between ignoring the infection and
making autoantibodies (see Fig. 38)
that may be damaging to its own tissues. Borrelia, another
spirochaete, has the property (found also with some viruses and protozoa) of
varying its surface antigens to confuse the host’s antibody-forming system. As
a result, waves of infection are seen (‘relapsing fever’).
Brucella may do the same.