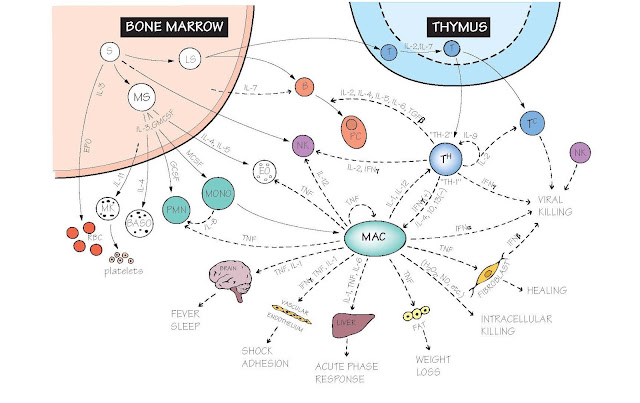
The Cytokine Network
In the previous chapter cytokines were introduced as a collection of
distinct molecules and receptors, but
with a bewildering spectrum of regulatory effects on immunity and immune
responses. Numerous cells can make one or several cytokines, depending on the circumstances.
Very few cytokines are confined to a single function (pleiotropy) and very few
functions rely on a single cytokine (redundancy). There are obvious advantages
in this arrangement, for example the chance loss of a single cytokine or
cytokine receptor gene would be unlikely to cause serious trouble – although
there are exceptions to this (see Fig. 33). The analogy has even been made with
language: one can communicate reasonably well with alphabets progessively
lacking individual letters, but there would come a point where all messages
would read the same. Many cytokines have related structures, and are thought to
have evolved via repeated gene duplication (see Chapters 46 and 47). Presumably
the present number of cytokines and
functions is what nature, through evolution, has found to be adequate without too much in the way of
unwanted effects. Interestingly, some cytokines (e.g. interferons) are highly
species-specific, others much less so.
The figure shows the combinations
of cytokines responsible for the main pathways of immune cell development,
differentiation, interaction and function, together with some of the side
effects that can result from over-activity. As knowledge has accumulated,
cautious attempts have been made to use cytokines in the clinic, although not
many have yet become standard therapeutical agents. In fact, the most dramatic
effects have come from blocking excessive cytokine activity, and both natural
inhibitors and soluble receptors are being extensively tried out. At present, the
amelioration of some cases of rheumatoid arthritis by anti-TNF antibodies is
probably the best-known example; some others are mentioned on the opposite page.
Bone marrow (see also Figs 4 and 15). Unlike most other tissues of the body, the number of each type of immune
cell varies greatly, depending on the amount of immune activity (hence the
white blood cell count is often used as an indicator of disease; see Fig. 44).
In addition, the turnover of cells in the immune system is very high (about
1010 neutrophils alone are formed and die each day in a healthy adult).
Cytokines have a major role in regulating the proliferation, differentiation
and commitment of immune and other blood cells from multipotent stem cells in
the bone marrow. Some of these cytokines (stem cell factor, IL-7, IL-11) are
made by bone marrow stromal cells, others (IL-3, IL-5, granulocyte macrophage
colony-stimulating factor [GM-CSF], macrophage colony-stimulating factor
[M-CSF], granulocyte colony-stimulating factor [G-CSF]) by T cells, macrophages
or other tissues of the body. GCSF, which stimulates the development of
granulocytes, is used to boost the production of neutrophils after bone marrow
transplantation.
Immature B lymphocytes differentiate
and proliferate in the bone marrow independently of antigen, in response to
IL-7 and other cytokines. Once mature B cells have recognized their specific
antigen, their differentiation into memory cells and plasma (antibody-
producing) cells is controlled by cytokines from T helper (Th) cells such as
IL-2, IL-4 and IL-6. Cytokines are particularly involved in Ig class switching,
e.g. IL-4 for IgE, IL-5 and TGF-β for IgA.
Thymus Here T cells mature and are selected for MHC
and antigen specificity (see also Figs 16 and 17). Thymic stromal cells produce
cytokines of which IL-7 is the best known, but cell surface molecules known as Notch
also play a part. The older concept of thymus hormones (e.g.
thymosin) is still debatable.
T lymphocytes both secrete IL-2 and express receptors for it
so that they can stimulate their own proliferation (autocrine); this molecule
was formerly known as T-cell growth factor (TCGF). Different T-cell subsets go
on to predominantly secrete different cytokines: Th1 cells activate macrophages
via IFNγ, Th2 cells regulate B cells as described above, and the newly
recognized Th17 subset activates polymorphonuclear leucocytes (PMN) via IL-17.
Several TREG subsets have been described, all with the ability to suppress Th
cells. Interestingly, the expression of very high levels of one chain of the
IL-2 receptor, CD25, is characteristic of regulatory T cells, and IL-2
deficiency leads preferentially to a deficit in regulatory T cells. The
cytokines TGF-β and IL-10 mediate some of these activities. The differentiation
of these different T subsets is itself regulated by cytokines: IL-12 secreted
by dendritic cells, for example, favours TH1 development, IL-4 from mast cells
favours TH2, and IL-23 and TGF-β favour TH17
cells.
Macrophages act as key sentinels found within all organs of
the body, releasing cytokines on contact with microbes which then initiate
immune responses. Macrophages are the main source of the inflammatory cytokines
TNF, IL-1 and IL-6. These cytokines are released into the blood stream, and act
systemically, controlling the vasculature, the hypothalamus, muscle and liver.
The antiviral cytokines IFNα and IFNβ are produced in very high amounts by a
rare blood cell, the plasmacytoid dendritic cell.
Natural killer (NK) cells (see also Fig. 15). Their main function is to
kill virus-infected and some tumour cells, but they are also important sources
of IFNγ. Several cytokines are involved in their development (IL-12, IL-15)
and activation (IL-12, IL-18, IFNα,β).
Microbial killing IFNα and β have a major role early in virus
infections, both by damage to viral RNA and by enhancing MHC class I
expression. Macrophage-derived TNF, IL-1 and IL-6 initiate the acute phase
response, fever and, via IFNγ, the killing of intracellular microbes. In
helminth infections Th2 cell-derived IL-4 and IL-5 are responsible for IgE
production and eosinophilia, respectively.
Inflammation Here changes to vascular endothelium are
critical, and TNF has a leading role, stimulating the increased production of
adhesion molecules on the inner surface of blood vessels (see Fig. 7), the
secretion of chemokines and the autocrine activation of macrophages. In severe
infections or injuries, excessive TNF can get into the circulation, leading to
shock and multiple organ damage. Type I acute inflammation (hypersensitivity)
is interesting in that several relevant genes (IL-3, IL-4, IL-5, IL-9, IL-13)
lie together on chromosome 5q (see Fig. 47), which is known to be a
susceptibility locus for allergies.
Leucocyte migration Most leucocytes are very motile, not only
circulating in blood, but leaving the blood, crossing the endothelium and
migrating though lymphoid and non-lymphoid tissues. The chemokines have a key
role in chemotaxis, the regulation of leucocyte traffic (e.g. attracting neutrophils, lymphocytes and
monocytes to inflammatory sites) and the maintenance of the correct lymphoid
architecture. The manipulation of chemokine pathways for therapy has so far
been limited, partly because many of the chemokines have multiple and
overlapping functions, and can bind to many different receptors.
Cytokines in therapy Early enthusiasm for cytokine treatment of
tumours and infections, particularly HIV, has been dampened by severe side
effects and, in many cases, ineffectiveness. At present the main cytokines in
clinical use are IFNα for viral hepatitis, IFNα and IL-2 for certain tumours,
notably renal, and IFNβ for treatment of multiple sclerosis. More dramatically
successful is the use of cytokine antagonists (generally in the form of
monoclonal antibodies) to control chronic inflammatory diseases, e.g. anti-TNF
in rheumatoid arthritis. Anti-TNF is also under study for osteoarthritis, gout
and heart failure. Disappointingly, it is only moderately beneficial in septic
shock. An alternative approach is to use soluble receptors to block cytokine
activity; the IL-1 receptor is the leading example.