Heart Failure
Chronic heart failure is
a complex and progressive disorder that occurs when the heart is incapable of
generating sufficient cardiac output (CO) to meet the demands of the body.
Initially, compensatory mechanisms may allow adequate CO to be maintained at
rest but not during exercise (exercise intolerance). Eventually CO
cannot be maintained at rest (decompensation); this can be precipitated
by acute illness (e.g. influenza), stress or drugs (e.g. NSAIDs). Chronic heart failure is
predominantly a disease of old age. It occurs in ∼2% of patients under 50 years, but >10% over 65; 5-year survival
is <50%. Acute heart failure describes a sudden loss of cardiac
function, for example acute coronary syndrome (see Chapter 44).
It may cause pulmonary congestion and oedema (see below) and cardiogenic shock
(see Chapter 31).
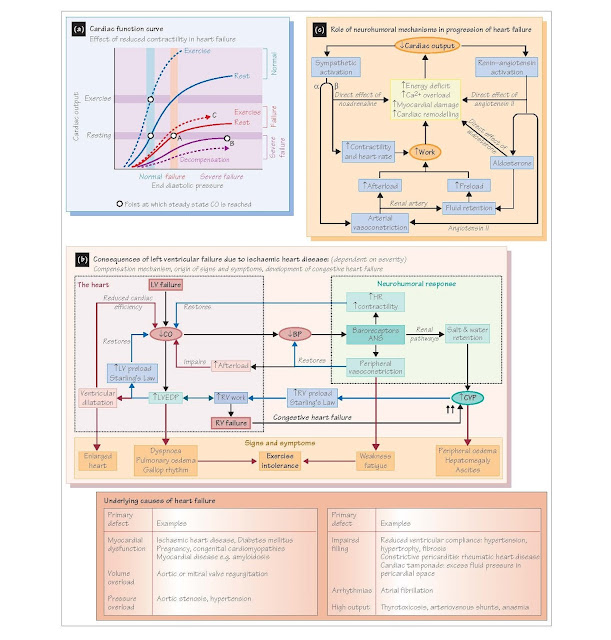
The most common cause (∼70% cases) is impaired ventricular contraction with an ejection
fraction <45% (systolic failure; Figure 46a), generally a
consequence of ischaemic heart disease (IHD). Diastolic failure is due
to impaired filling, caused by reduced ventricular compliance (flexibility;
e.g. fibrosis, hypertrophy), restriction (e.g. pericarditis) or impaired
relaxation (see below). Ejection fraction may be normal or increased. Systolic
failure is generally accompanied by diastolic failure, while the latter can
occur alone. Both involve increased filling pressures, so have similar clinical
manifestations.
As IHD generally affects the left ventricle, left
heart failure is most common, and is associated with dyspnoea (breathlessness),
an enlarged heart and fatigue (see below). Right heart failure
may result from chronic lung disease (cor pulmonale), pulmonary hypertension or embolism, and valve disease, but
usually it is secondary to left heart failure (congestive or biventricular
heart failure) (Figure 46b). Central venous pressure
(CVP) is greatly increased, with consequent jugular venous distension, swelling
of the liver (hepatomegaly), peripheral oedema and peritoneal
fluid accumulation (ascites). High output failure occurs when a
healthy heart is unable to meet grossly elevated demands for output due to
anaemia or a drastically reduced peripheral resistance (e.g. septic shock).
Pathophysiology
The pathophysiology of chronic heart failure is largely
a consequence of mechanisms that compensate for reduced cardiac function.
Impaired cardiac function causes accumulation of venous blood and thus raised
filling pressures, so CO increases as a consequence of Starling’s law (Figure
46a,b; see Chapter 17). Neurohumoral mechanisms are activated by the baroreceptor
reflex (Figure 46c; see Chapter 28), and the autonomic nervous system and
circulating catecholamines stimulate increases in heart rate and contractility,
arterial vasoconstriction (raises TPR) and venoconstriction (raises CVP) (see
Chapters 12 and 17). Sympathetic stimulation of renal granular cells and
reduced renal perfusion cause release of renin, and consequently angiotensin
II and aldosterone; vasopressin (antidiuretic hormone, ADH) also
increases. These cause renal sodium and water retention and so elevate blood
volume and CVP (and thus CO through Starling’s law) (see Chapter 29).
Angiotensin II and vasopressin also increase TPR. In mild disease these
mechanisms can maintain CO and blood pressure without overt symptoms. However,
end-diastolic pressure (EDP) and volume (EDV) are always elevated (Figure 46a,
A) so ejection fraction is reduced, an early sign of heart failure.
As cardiac function declines, CO can only be maintained
by an ever-increasing CVP and heart rate (Figure 46a, B), fostering further myocardial
damage (see below). This vicious circle drives a relentless decay towards
decompensation and death. Although adequate CO may be maintained at rest even
in quite severe failure, this is at the expense of greatly increased venous
pressures as the function curve flattens and Starling’s law becomes less
effective (Figure 46a, A,B; see Chapter 17). High venous pressures underlie
most signs and symptoms of heart failure.
Consequences of compensation (Figure 46b)
Initially, symptoms only appear during exertion, which
exacerbates the rise in venous pressures (Figure 46a, C); this limits the
ability to exercise (exercise intolerance). Any increase in contractility
and heart rate during exercise is small because they are already strongly
stimulated at rest, and in late disease β-adrenoceptor density and sensitivity
are reduced. Dyspnoea on exertion is often the first symptom of left
heart failure. It is caused by pulmonary congestion due to the raised
pulmonary venous pressure, making the lungs stiffer and so promoting the
sensation of breathlessness. Redistribution of blood to the lungs on lying down
or during sleep can instigate dyspnoea (orthopnoea; paroxysmal
nocturnal dyspnoea), and in severe failure and decompensation pulmonary
oedema, when fluid enters the alveoli. This is a life-threatening condition
causing extreme dyspnoea and hypoxaemia.
A high CVP similarly causes peripheral oedema (see
Chapter 21), hepatomegaly and ascites, common features of right
and congestive heart failure. High EDP eventually lead to cardiac
dilation and a greatly enlarged heart (see below), and is associated with
an S3/S4 gallop rhythm (see Chapter 16). In more
severe disease diversion of blood flow from skeletal muscle and non-essential
tissues leads to weakness and fatigue, and contributes to
exercise intolerance.
Myocardial dysfunction and remodelling
Chronic heart failure is characterized by progressive
cardiac dysfunction, accompanied by myocardial remodelling.
Compensation forces an already compromised heart to work
harder. This leads to energy deficit, dysfunction of ATP-dependent
transporters (e.g. Ca2+-ATPases and Na+ pump) (see
Chapters 10 and 12), and consequent Ca2+ overload (Figure
46c). This impairs relaxation and fosters lengthening of the action potential
(e.g. acquired long QT syndrome; see Chapter 54) and generation of arrhythmias,
a major cause of sudden death. Mitochondrial dysfunction worsens the energy
deficit. Oxidative stress, and cytokines promote further damage,
structural alterations and apoptosis (programmed cell death). Myocardial
remodelling is potentiated by direct action of noradrenaline, angiotensin II
and aldosterone (Figure 46c).
Dilatation reduces
cardiac efficiency, as pressure in a sphere is proportional to wall
tension (i.e. myocardial force) divided by radius (Law of Laplace). Large
dilated hearts therefore have to contract harder in order to develop the same
pressure as smaller hearts.
Cardiac dilatation must not be confused with hypertrophy,
where cardiac myocytes grow larger and ventricular wall thickness increases in
response to a sustained increase in afterload (e.g. hypertension, aortic
stenosis). Hypertrophy is not usually associated with IHD. Although force is
increased, the thicker ventricle is less compliant, which impedes
filling and contributes to diastolic failure. Capillary density is
reduced, lowering coronary reserve (difference between maximum and
resting coronary flow), so myocardial perfusion may be limited. Changes in contractile
protein isoforms (myosin, tropomyosin) decrease contraction velocity and
contractility. Gross hypertrophy may physically impair valve operation.