Pathophysiology Of Acute Myocardial Infarction
Infarction is tissue death caused by
ischaemia. Acute myocardial infarction (MI) occurs when localized
myocardial ischaemia causes the development of a defined region of necrosis.
MI is most often caused by rupture of an atherosclerotic lesion in a coronary
artery. This causes the
formation of a thrombus that plugs the artery, stopping it from supplying blood
to the region of the heart that it supplies.
Pivotal studies by DeWood and colleagues showed that coronary
thrombosis is the critical event resulting in MI. Of patients presenting
within 4 h of symptom onset with ECG evidence of transmural MI, coronary
angiography showed that 87% had complete thrombotic occlusion of the
infarct-related artery. The incidence of total occlusion fell to 65% 12–24 h after
symptom onset due to spontaneous fibrinolysis. Fresh thrombi on top of ruptured
plaques have also been demonstrated in the infarct-related arteries in patients
dying of MI.
Mechanisms and consequences of plaque rupture
Coronary plaques that are prone to rupture are typically
small and non-obstructive, with a large lipid-rich core covered by a thin fibrous
cap. These ‘high-risk’ plaques typically contain abundant macrophages and
T lymphocytes which are thought to release metalloproteases and cytokines
that weaken the fibrous cap, rendering it liable to tear or erode due to
the shear stress exerted by the blood flow.
Plaque rupture reveals subendothelial collagen, which
serves as a site of platelet adhesion, activation and aggregation. This results
in:
1 release of substances such as thromboxane A2 (TXA2),
fibrinogen, 5-hydroxytryptamine (5-HT), platelet activating
factor and adenosine diphosphate (ADP), which further promote
platelet aggregation.
2 Activation of the clotting cascade, leading to fibrin formation and
propagation and stabilization of the occlusive thrombus.
The endothelium is often damaged around areas of
coronary artery disease. The resulting deficit of antithrombotic factors such
as thrombomodulin and prostacyclin enhances thrombus formation.
In addition, the tendency of several platelet-derived factors (e.g. TXA2,
5-HT) to cause vasoconstriction is increased in the absence of
endothelial-derived relaxing factors. This may promote the development of local
vasospasm, which worsens coronary occlusion.
Sudden death and acute coronary syndrome onset show a circadian
variation (daily cycle), peaking at around 9 a.m. with a trough at around
11 p.m. Levels of catecholamines peak about an hour after awakening in the
morning, resulting in maximal levels of platelet aggregability, vascular tone,
heart rate and blood pressure, which may trigger plaque rupture and thrombosis.
Increased physical and mental stress can also cause MI and sudden death,
supporting a role for increases in catecholamines in MI pathophysiology.
Furthermore, chronic β-adrenergic receptor blockade abolishes the circadian rhythm
of MI.
Autopsies of young subjects killed in road accidents
often show small plaque ruptures in susceptible arteries, suggesting that
plaque rupture does not always have pathological consequences. The degree of
coronary occlusion and myocardial damage caused by plaque rupture probably
depends on systemic catecholamine levels, as well as local factors such as
plaque location and morphology, the depth of plaque rupture and the extent to
which coronary vasoconstriction occurs.
Severe and prolonged ischaemia produces a region of
necrosis spanning the entire thickness of the myocardial wall. Such a transmural
infarct usually causes ST segment elevation (i.e. STEMI; see Chapter 45).
Less severe and protracted ischaemia can arise when:
·
Coronary occlusion is followed
by spontaneous reperfusion
·
The infarct-related artery is
not completely occluded
·
Occlusion is complete, but an
existing collateral blood supply prevents complete ischaemia
·
The oxygen demand in the
affected zone of myocardium is smaller.
Under these conditions, the necrotic zone may be mainly
limited to the sub endocardium, typically causing non-ST segment elevation MI.
The classification of acute MI according to the presence
or absence of ST segment elevation is designed to allow rapid decision-making
concerning whether thrombolysis should be initiated (see Chapter 43). This
classification replaces the previous one, based on the presence or absence of Q
waves on the ECG, which was less useful for guiding immediate therapy.
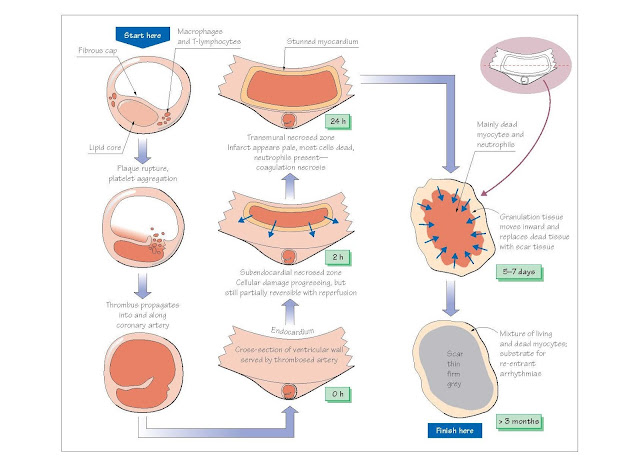
Evolution of the infarct
Both infarcted and unaffected myocardial regions undergo
progressive changes over the hours, days and weeks following coronary
thrombosis. This process of postinfarct myocardial evolution leads to the
occurrence of characteristic complications at predict- able times after the
initial event (see Chapter 45).
Ischaemia causes an immediate loss of contractility in
the affected myocardium, a condition termed hypokinesis. Necrosis starts
to develop in the subendocardium (which is most prone to ischaemia; see Chapter
2), about 15–30 min after coronary occlusion. The necrotic region grows outward
towards the epicardium over the next 3–6 h, eventually spanning the entire
ventricular wall. In some areas (generally at the edges of the infarct) the
myocardium is stunned (reversibly damaged) but will eventually recover
if blood flow is restored. Contractility in the remaining viable myocardium
increases, a process termed hyperkinesis.
A progression of cellular, histological and gross
changes develop within the infarct. Although alterations in the gross
appearance of infarcted tissue are not apparent for at least 6 h after the
onset of cell death, cell biochemistry and ultrastructure begin to show
abnormalities within 20 min. Cell damage is progressive, becomingly increasingly
irreversible over about 12 h. This period there- fore provides a window of
opportunity during which percutaneous coronary intervention (PCI) or
thrombolysis leading to reperfusion may salvage some of the infarct (see
Chapter 43).
Between 4 and 12 h after cell death starts, the
infarcted myocardium begins to undergo coagulation necrosis, a process
characterized by cell swelling, organel lebreak down and protein denaturation. After about 18 h, neutrophils (phagocytic
lymphocytes) enter the infarct. Their numbers reach a peak after about 5 days,
and then decline. After 3–4 days, granulation tissue appears at the
edges of the infarct zone. This consists of macrophages, fibroblasts,
which lay down scar tissue, and new capillaries. The infarcted myocardium
is especially soft between 4 and 7 days, and is therefore maximally prone to rupturing.
This event is usually fatal, may occur at any time during the first 2 weeks,
and is responsible for about 10% of MI mortality. As the granulation tissue
migrates inward toward the centre of the infarct over several weeks, the
necrotic tissue is engulfed and digested by the macrophages. The granulation
tissue then progressively matures, with an increase in connective (scar) tissue
and loss of capillaries. After 2–3 months, the infarct has healed, leaving a
non-contracting region of the ventricular wall that is thinned, firm and pale
grey.
Infarct expansion, the
stretching and thinning of the infarcted wall, may occur within the first day
or so after an MI, especially if the infarction is large or transmural, or has
an anterior location. Over the course of several months, there is progressive
dilatation, not only of the infarct zone, but also of healthy myocardium. This
process of ventricular remodelling is caused by an increase in end diastolic wall
stress. Infarct expansion puts patients at a substantial risk for the
development of congestive heart failure, ventricular arrhythmias and free wall
rupture.