Ventricular Tachyarrhythmias And Nonpharmacological Treatment Of
Arrhythmias
Tachyarrhythmias originating in the ventricles are
most often associated with ischaemic heart disease and primary or
secondary heart failure (i.e. dilated cardiomyopathies). They are common during
and up to 24 h after acute myocardial infarction (MI), when increases in
sympathetic activity and extracellular [K+] as well as slowed conduction favour
their initiation. Such peri-infarction arrhythmias may be immediately
life-threatening, and indeed the vast majority of deaths associated with MI are
caused by ventricu- lar fibrillation occurring before the individual reaches
the hospital. If survived, these arrhythmias generally do not recur and are not
associated with a subsequent increased risk over and above that conferred by
the MI itself. Subsequently, however, the border zone of the healed infarct
scar may serve as a substrate for the development of dangerous re-entrant
ventricular tachyarrhythmias which can recur or become incessant weeks to years
after the MI. Their seriousness and prognostic significance are related to the
extent of cardiac damage and impairment of ventricular function that has been
sustained. These late arrhythmias themselves confer an additional risk of
death, and must be treated either with drugs or with an implantable
defibrillator (see below). Ventricular tachyarrhythmias can also be
associated with cardiomyopathy, and valvular and congenital heart disease,
although idiopathic varieties may occur in structurally normal hearts.
Specific ventricular
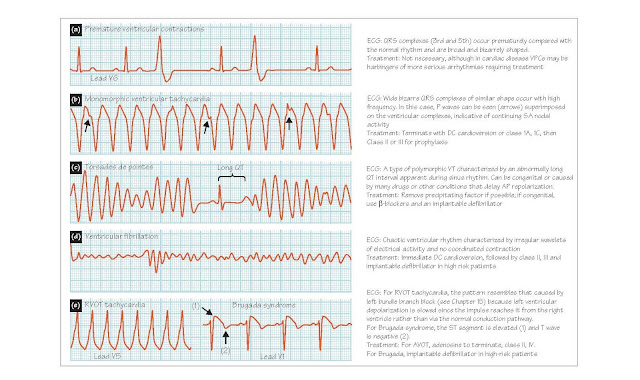
tachyarrhythmias Premature ventricular contractions (PVCs) are caused by a ventricular ectopic focus
and can occur randomly or following every (bigeminy; Figure 50a) or
every second (trigeminy) normal beat. Because depolarization is
initiated at a site within ventricular muscle, it spreadsthroughouttheventriclesmoreslowly
thannormalimpulses which are distributed rapidly by the specialized
His–Purkinje conduction system. Thus, the QRS complex is broad and abnormally
shaped. PVCs may be of no prognostic consequence, but can predispose to more
serious arrhythmias if they develop during or after MI, and/or occur during the
T wave of the preceding beat.
Ventricular tachycardia (VT) originates in the ventricles, and is defined
as a run of successive ventricular ectopic beats occurring at a rate of >100
beats/min (usually 120–200 beats/min). VT is classified as non-sustained or
sustained based on whether it lasts for >30 s. Depending on the heart
rate, VT can cause symptoms such as syncope, angina and shortness of breath,
and if sustained can compromise cardiac pumping, leading to heart failure and
death. VT can also deteriorate into ventricular fibrillation (see below),
particularly with a heart rate of >200 beats/min.
The ECG in VT demonstrates high
frequency, bizarrely shaped QRS complexes which are abnormally broadened
(>120 ms in duration). Normal atrial activation may continue to be driven by
the SAN (Figure 50b), or the abnormal ventricular pacemaker may cause atrial
tachycardia via retrograde impulses traversing the AVN. The configuration of
the QRS complex can be used to classify VT into two broad categories. In monomorphic
VT (Figure 50b), the QRS complexes all have a similar configuration and the
heart rate is generally constant, whereas in polymorphic VT both the QRS
configuration and the heart rate vary continually. Mono-morphic VT generally
indicates the presence of a stable re-entrant pathway, the substrate for which
is typically an MI-related scar (see Chapter 48). Polymorphic VT is thought to
be caused by multiple ectopic foci or re-entry in which the circuit pathway is
continually varying, and most often occurs during or soon after an MI.
Torsade de pointes (‘twisting of the points’) is a type of
polymorphic VT in which episodes of tachycardia, which may give rise to
fibrillation and sudden death, are superimposed upon intervals of bradycardia,
during which the QT interval (indicative of the ventricular action potential
duration) is prolonged (Figure 50c). During the tachycardia, the ECG has a
distinctive appearance in which the amplitude of the QRS complexes alternately
waxes and wanes. Torsade de pointes may be caused by drugs or conditions that
delay ventricular repolarization (e.g. class IA and III antiarrhythmics,
hypokalaemia, hypomagnesaemia). It is also associated with congenital long
QT (LQT) syndrome, which can be caused by mutations in KvLQT1 or HERG,
genes coding for cardiac K+ channels mediating repolarization, or SCN5A,
the gene coding for the cardiac Na+ channel. In congenital LQT syndrome, torsades
de pointes is often triggered by sympathetic activity (e.g. caused by stress),
which may give rise to early or delayed afterde-polarizations, and may also
involve functional re-entry mediated by spiral waves of depolarization (see
Chapter 48).
Ventricular fibrillation (VF) is achaotic ventricularrhythm(Figure 50d)
incompatible with a cardiac output which will rapidly cause death unless the
patient is resuscitated. VF may follow episodes of VT or acute ischaemia, and
frequently occurs during MI. It is the main cause of sudden death, which is
responsible for ∼10% of all mortality. VF is generally associated
with severe underlying heart disease, including ischaemic heart disease and
cardiomyopathy.
Focal VT and fascicular tachycardia are forms of VT
that are idiopathic (i.e. can occur in structurally normal hearts). Focal VT
most commonly originates in the right ventricular outflow tract (RVOT
tachycardia; Figure 50e, left) and is associated with increases in sympathetic
activity. This is thought to raise intracellular [cyclic AMP] and therefore
[Ca2+]i, initiating delayed afterdepolarizations. Fascicular
tachycardia may in some cases be caused by a re-entrant circuit involving the
Purkinje system. Idiopathic VTs generally have a good prognosis, and can
usually be successfully eliminated with radiofrequency catheter ablation (see
below). VF occasionally occurs idiopathically, for example in people with LQT
syndrome or Brugada syndrome (Figure 50e, right). This latter condition
is associated with ion channel mutations (e.g. in SCN5A) which shorten
the action potential in epicardial but not endocardial cells of the right
ventricle, a situation favouring the development of re-entry.
Direct current (DC) synchronized
cardioversion allows rapid cardioversion (reversion to sinus rhythm) of haemodynamically unstable VT and SVT.
Shocks of 50–200 J are delivered in synchrony with the R wave of the QRS
complex to the anaesthetized patient via adhesive defibrillator pads placed
below the right clavicle and over the apex of the heart.
Radiofrequency catheter ablation
(RCA) has assumed a central role
in treating many types of arrhythmias. In RCA, the pathways or focally
automatic sites causing certain tachyarrhythmias are ablated (destroyed) by
focal heating delivered via a catheter. The catheter is inserted through a vein
and the tip is located at the surface of the endocardium at the site of the
abnormality. Radi- ofrequency energy is delivered to the tip and dissipated to
a large indifferent plate, usually over the back. The tip temperature is set to
60–65°C, resulting in a lesion 8–10 mm in diameter and of a similar depth. This
technique is curative in >90% of certain supraventricular arrhythmias. RCA is
also increasingly being used to treat VT when an appropriate target site (e.g.
a slowly-conducting ‘isthmus’ in a myocardial scar) can be identified.
Although seldom causing complications,
RCA of sites very close to the AV node can potentially cause inadvertent AV
nodal damage and therefore permanent block, requiring pacemaker implantation.
This can be avoided using cryoablation, in which the catheter tip is
cooled rather than heated. Cooling the tip briefly to −30°C causes a focal
block of electrical activity that is reversible and so cannot cause permanent
damage. If this stops the arrhythmia without causing undesirable effects the
tip is then further cooled to −60°C, which causes a permanent lesion and
ablation of the abnormal rhythm.
Implantable defibrillators consist of a generator connected to electrodes
placed transvenously in the heart and superior vena cava. A sensing circuit
detects arrhythmias, which are classified as tachycardia or fibrillation on the
basis of rate. The treatment algorithm is either as burst pacing, which can
terminate VT with a high degree of success, or by the delivery of a shock at up
to 40 J, which can cardiovert VT and VF. Shock delivery is between an electrode
in the right ventricle and another in the superior vena cava or to the body of
the generator (active can). Refinements in detection allow the distinction of
supraventricular and ventricular arrhythmias, so that several tiers of
progressively more aggressive therapy can be set up. The AVID study reported in
1997 that in patients with malignant ventricular arrhythmia, this approach
improved survival by 31% over 3 years compared with anti-arrhythmic drug
therapy (mainly amiodarone).
Electronic pacemakers can be used either temporarily or permanently to
initiate the heart beat by imposing repeated cardiac depolarizations. Temporary
pacing is generally accomplished using a catheter-tipped electrode introduced
transvenously and provides for the rapid treatment of bradycardias. A temporary
pacemaker can also be used to terminate a persistent arrhythmia by pacing the
heart at a rate somewhat faster than that of the arrhythmia; sinus rhythm is
often restored when this overdrive pacing is stopped. Permanent
pacemakers are usually implanted to treatbradycardias, forexample due to AV
block or sick sinus syndrome (see Chapter 13). The pacemaker is implanted under
the skin on the chest, and stimulates the heart through leads introduced into
the heart transvenously, usually through the subclavian vein. Contemporary
pacemakers are able to pace both the atria and ventricles to maintain AV
synchronization, and to adjust the pacemaking frequency to respond to changes
in physical activity by sensing parameters such as respiration and the interval
between the stimulated depolarization and the T wave, a measure of sympathetic
nervous system activity.