MYELOMA NEPHROPATHY
Multiple
myeloma is a malignant disorder of plasma cells that features clonal
proliferation of a single immunoglobulin-producing cell. These plasma cell
clones hypersecrete monoclonal (M) proteins, which can be either intact
immunoglobulins, usually of the IgG or IgA type, or free or light chains
(known as Bence-Jones proteins). The disease is generally diagnosed during the
sixth decade of life, and up to half of patients will experience renal
complications. Renal disease typically occurs as a result of the hypersecretion
of free light chains, which have a direct toxic effect on the kidneys; however,
urate nephropathy and direct plasma cell infiltration of the renal parenchyma
can occur as well.
PATHOPHYSIOLOGY
Plasma cells
normally release a modest excess of free light chains that are excreted in
urine at the rate of 10 to 30 mg/day. These proteins are filtered at the
glomerulus, and the majority are reabsorbed by proximal tubular cells and
subsequently catabolized.
In multiple
myeloma, light chains may be present in such overwhelming excess that they
overcome the reabsorptive capacity of the nephrons. The high concentrations of
light chains in the tubular lumina may in turn lead to a phenomenon known as
“cast nephropathy.” Injury occurs through two mechanisms. First, light chains
form obstructive casts that can cause acute or chronic renal failure. Second,
light chains accumulate in proximal tubule cells because of resistance to
degradation, which leads to tubular epithelial cell injury (and, in turn, impaired
proximal light chain reabsorption and increased delivery to the distal tubule).
The
formation of light chain casts is dependent on several factors. First, the
light chains must reach a threshold concentration. Second, the light chains
must bind to Tamm-Horsfall protein, normally produced in the thick ascending
limb. Light chain casts are thus usually found in the distal tubule because of
the increased light chain concentration in this segment (secondary to fluid
reabsorption in more proximal segments), as well as the presence of
Tamm-Horsfall protein.
Several
factors can precipitate cast nephropathy in a patient with myeloma. Many of
these factors either reduce glomerular filtration rate (GFR) or slow the rate of
distal tubular fluid delivery, thereby raising the concentration of tubular
light chains and favoring cast formation. For example, NSAIDs, ACE inhibitors,
intravenous contrast, and infection can precipitate cast nephropathy, likely by
decreasing GFR. Likewise, diarrheal illness, hypercalcemia, and diuretics are
precipitants that likely act by causing volume depletion.
Less
commonly, the M proteins may deposit in the glomerulus, where they disrupt the
protein filtration barrier (e.g., light chain deposition disease, amyloidosis, and immunotactoid
glomerulonephritis). In addition, the light chains may sometimes cause
extensive damage to the proximal tubule, resulting in more generalized
reabsorption defects (renal Fanconi syndrome).
PRESENTATION
AND DIAGNOSIS
Nearly 50%
of patients with myeloma cast nephropathy have acute kidney injury; the
remaining cases are either subacute or chronic. A typical presentation in a patient without known
myeloma is several weeks of oliguria, weakness, fatigue, lethargy, and lower
extremity edema with newly diagnosed severe renal insufficiency. In cotrast, a
patient with a known myeloma diagnosis will often be noted to have an
asymptomatic rise in creatinine on routine laboratory evaluation. Some of the
known precipitants, listed previously, may be noted in the recent clinical
history.
On further evaluation, patients will be
found to have bland urine sediment, minimal dipstick proteinuria, and
subnephrotic-range proteinuria on a quantitative collection. The dipstick
measurement of proteinuria is generally unremarkable because it detects only
albumin, whereas these patients excrete large quantities of light chains.
Photometry of a urine specimen after the addition of a precipitant such as
sulfosalicylic acid or tri- chloroacetic acid, however, will reveal the presence
of all urine proteins. More than a gram of protein on a quantitative
photometric urine specimen with a negative dipstick for albumin is suggestive
of paraproteinuria. Immunofixation electrophoresis of serum and urine should be
performed to confirm and identify the paraproteins.
In contrast,
a strongly positive dipstick and nephroticrange proteinuria in the setting of
myeloma suggests AL amyloidosis or light chain deposition disease, since these
conditions cause glomerular injury that allows albumin to enter the urinary
space.
The
definitive diagnosis of myeloma depends on the observation of a monoclonal
protein on serum or urine protein electrophoresis, demonstration of 10% or more
clonal plasma cells on bone marrow biopsy, and evidence of organ damage. Recently,
the ability to quantify free light chains has provided a more sensitive
diagnostic modality. In multiple myeloma and other monoclonal gammopathies,
overexpression ofκ or λ restricted light chains
causes the κ:λfree light chain ratio
to become abnormal.
Although
cast nephropathy is highly probable in a patient with confirmed myeloma who has
renal failure, a bland sediment, and minimal albuminuria, a definitive diagnosis
requires renal biopsy.
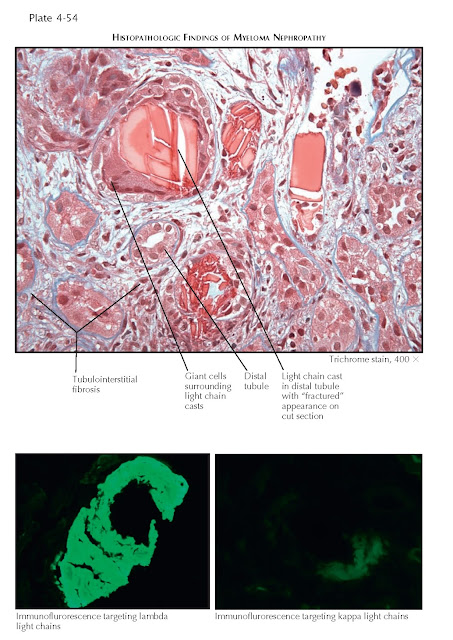
The exact
indications for renal biopsy are controversial. If one is performed,
characteristic findings include intratubular casts that have a “fractured”
appearance, with adjacent reactive cells that include multinucleated giant
cells. On immunofluorescence, these casts stain only for κ or λ light chains, which
corresponds to the abnormal light chain. Patients with more chronic disease may
have a variable degree of tubulointerstitial fibrosis.
TREATMENT
Treatment of
myeloma cast nephropathy centers on volume expansion, which reduces
intratubular cast concentration, as well as chemotherapy (and sometimes
plasmapheresis), which reduces serum free light chain concentration. The role
of plasmapheresis is controversial. One study of patients with biopsy-proven myeloma
cast nephropathy found that plasmapheresis led to a 50% reduction in serum
creatinine concentration, as well as dialysis independence, in those who experienced a more than 50%
reduction in the serum free light chain concentration. Other studies, however,
have shown no benefit. Dialysis is offered to patients who have advanced renal
failure as a supportive measure, but it does not influence the course of the
disease.
PROGNOSIS
Survival in
patients with multiple myeloma is inversely correlated with serum creatinine
concentration at presentation,
as shown in a study from the 1980s that found a median survival of 44, 18, and
4.3 months in patients with creatinine less than 1.4, 1.4 to 2.0, and greater
than 2.0 mg/dL, respectively. The potential for improvement of renal function
in response to treatment correlates best with the degree of tubulointerstitial
fibrosis and tubular atrophy on biopsy. Recovery of renal function has been
known to occur in patients who require dialysis, occuring up to 3 months after
dialysis onset. a more than 50%
reduction in the serum free light chain concentration. Other studies, however,
have shown no benefit. Dialysis is offered to patients who have advanced renal
failure as a supportive measure, but it does not influence the course of the
disease.