RENAL TRANSPLANTATION
The concept of replacing a diseased human organ with tissue from a living or deceased person has existed since ancient times. The different kinds of transplanted tissue include an autograft (tissue from the recipient), an isograft (tissue from an individual with the same genotype, such as a monozygotic twin), an allograft (tissue from a genetically disparate individual from the same species), and a xenograft (tissue from a different species).
As early as
1916, Little and Tyzzer articulated the important differences between these
graft types, stating “isografts succeed; allografts are rejected.” A century
later, the clinical practice of transplantation remains subject to these laws.
Nonetheless, the introduction of modern immunosuppression drugs has led to
dramatic improvements in allograft outcomes. As a result, kidney
transplantation has become a common intervention.
 |
| Plate 10-26 RECIPIENT OPERATION IN KIDNEY TRANSPLANTATION |
Unfortunately, only a small minority of the patients that would benefit from a kidney transplant ever receive one. There is an ever-growing waiting list 84,355 patients in the United States in 2010 that far exceeds the number of annual procedures. In 2009, 16,830 kidney transplants were performed: 10,442 from a deceased donor, and 6388 from a living donor.
Despite the
growing need for organs, the number of deceased organ donors per year has been
stagnant. Nonetheless, the annual number of kidney transplants continues to rise.
Much of this growth has been fueled by an increase in live donors, in large
part because of substantial improvements in the organ harvesting process, such
as the introduction of minimally invasive techniques.
INDICATIONS,
DONOR MATCHING, AND PREOPERATIVE EVALUATION
All patients
with either end-stage renal disease or advanced chronic kidney disease (stage 4
or 5) should be considered for renal transplantation. Those who can tolerate
the surgical and anesthetic risks, and who can safely be immunosuppressed after
the transplant, are potential candidates. Relative contraindications include uncorrectable
advanced cardiopulmonary disease,
cirrhosis, active malignancies, active infections, active substance abuse, and
inadequate social support.
Before
transplantation, the donor and recipient must be confirmed to have compatible
blood types. In addition, precautions must be taken to ensure immune
compatibility. Recipient serum must be tested against donor lymphocytes to
ensure the recipient does not have preformed antibodies to donor proteins. The
problematic alloantibodies are most commonly directed against donor major
histocompatibility complex (MHC) class I and class II antigens. MHC class I
antigens are expressed on most nucleated cells, albeit at variable levels,
whereas MHC class II antigens are expressed mainly on antigen presenting cells
(B lymphocytes, dendritic cells, and some endothelial cells). Thus recipient serum is
tested against donor lymphocytes, which contain both MHC antigens. A positive
crossmatch predicts a high likelihood of hyperacute or early rejection.
In the past, the human leukocyte antigen
genes (HLA) of both donor and recipient, which encode the MHC genes, were also
examined to determine the risk of later alloantibody production and delayed
graft rejection. Because of the efficacy of current immunosuppressive therapies,
however, there is a reduced need to ensure HLA matching between the donor and
recipient. Moreover, acute rejection episodes that do occur can usually be
effectively treated. Nonetheless, transplants between HLA-identical siblings
continue to yield the best long-term outcomes.
Kidney
transplants from living donors result in superior outcomes compared with those
where the kidney has been obtained from a deceased donor. Proinflammatory and
proapoptotic physiologic perturbations associated with death, as well as the
increased cold ischemic times associated with deceased donor transplantation,
account for this difference in outcome.
 |
| Plate 10-27 MECHANISM OF ACTION OF IMMUNOSUPPRESSIVE MEDICATIONS IN KIDNEY TRANSPLANTATION |
PROCEDURE
Whether
procured from a living or deceased donor, kidneys for transplantation are
flushed with cold (4° C) preservation solution until asanguinous. The use of
cold organ preservation allows for successful trans- plantation even with
extended ischemic times, which may exceed 48 hours. As a result, kidneys may be
shipped over long distances to reach the recipient. Nonetheless, it is
preferable to minimize ischemic times. Deceased donor kidneys may also be
placed on a specialized mechanical perfusion apparatus that has been shown to
decrease the incidence of delayed allograft function.
Before the
harvested organ can be transplanted, surgeons must perform a functional
assessment of the allograft and review the anatomic report of the procuring
surgeon to determine if there are any tumors, vascular or ureteral anomalies,
or traumatic injuries to the kidney that could preclude successful
transplantation.
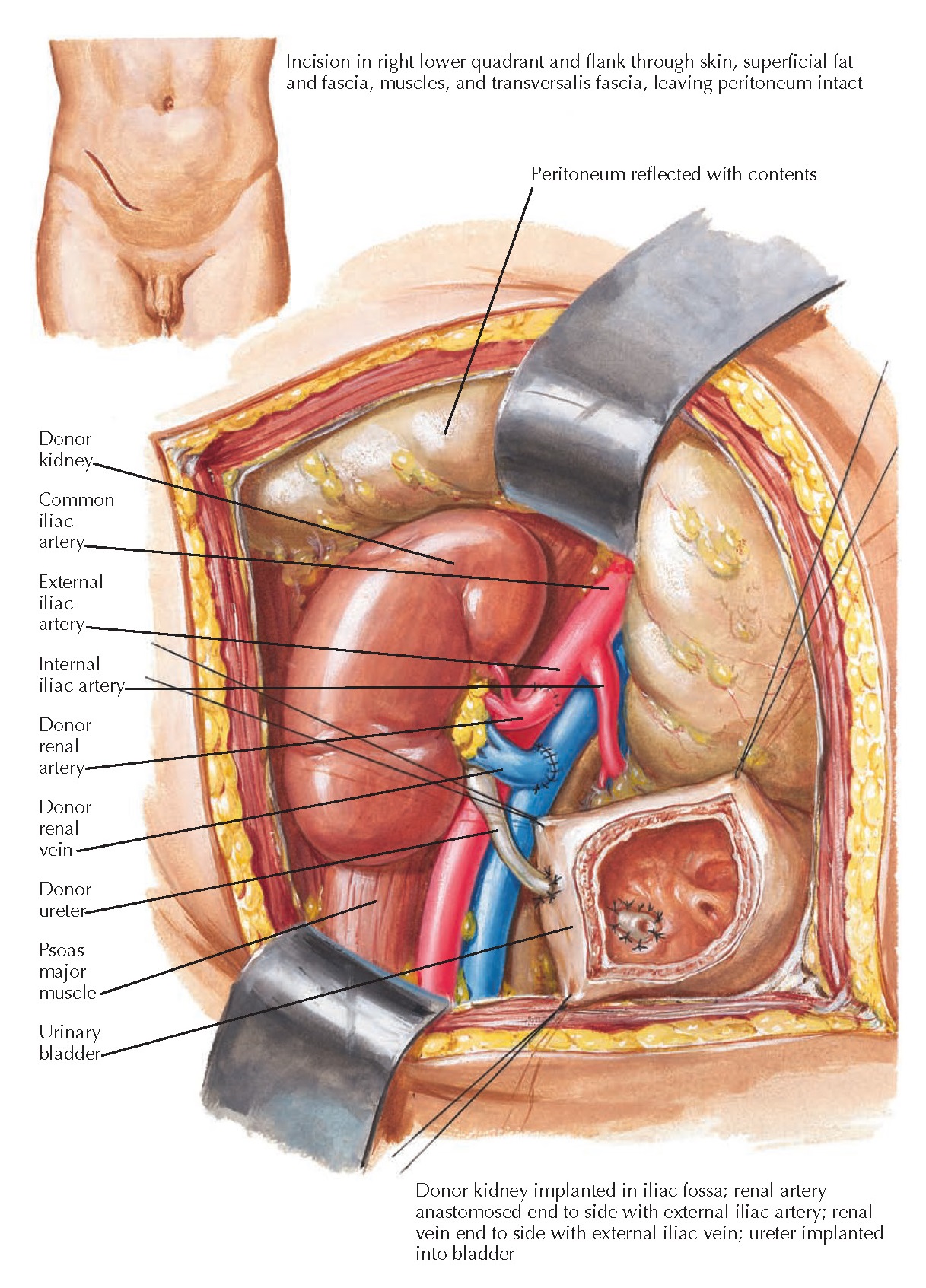
The
recipient operation is usually a heterotopic transplant, meaning the
recipient’s kidneys are left in place and the transplanted kidney is placed in
the iliac fossa, away from its normal anatomic position. The procedure is
performed through a Gibson or “hockey stick” incision in one of the lower quadrants of the abdomen. A renal
allograft can be implanted on either side, although many surgeons recommend
implanting a left kidney in the right iliac fossa and vice versa. The advantage
of this approach is that it positions the renal pelvis and ureter at the most
anterior aspect of the renal hilum, which facilitates access if reconstruction
is required at a later date.
The fascia
and muscle layers of the obliques and transversus are divided just lateral to
the edge of the rectus abdominis sheath. The superficial epigastric artery can be either
ligated and divided, or spared and mobilized medially. In females, the round
ligament is divided to mobilize the peritoneum, which is moved superiorly and
medially to uncover the external iliac artery and vein. In males, the spermatic
cord structures are preserved and mobilized medially to allow retraction of the
peritoneum from the abdominal wall.
Anastomosis
is performed between the renal allograft vein and external iliac vein, then
between the renal allograft artery and external iliac artery. The ends of the allograft vessels are sewn into the sides of the iliac vessels. After
the vascular reconstruction is complete, the graft is immediately reperfused.
The donor ureter is then anastomosed to the recipient’s bladder. The abdomen is
then closed, typically with no drains required.
IMMUNOSUPPRESSION
The fate of
the graft depends on the response of the recipient’s immune system. Thus
immunosuppression is critical. A combination of azathioprine and corticosteroids
was the first successful immunosuppression regimen, but the relative inefficacy
of this regimen, combined with the adverse effects of high-dose steroids, led
to poor outcomes in many patients. The introduction of cyclosporine in the
1980s brought about a dramatic improvement in outcomes and allowed for a
significant expansion of kidney transplantation.
In the modern
setting, there are several combinations of drugs that can be used to maintain
immunosuppression in renal transplant recipients. The most common regimen
includes a calcineurin inhibitor (tacrolimus, cyclosporine) and an
antimetabolite (mycophenolate, azathioprine) or sirolimus. Many centers also
include low-dose corticosteroids.
A delicate
balance must be maintained between avoidance of allograft rejection and side
effects, including opportunistic infections and malignancies. Infections in the
first month after transplant typically include postoperative surgical
infections, urinary tract infections, and pneumonias. At 1 to 6 months,
opportunistic infections such as Pneumocystis pneumonia and CMV
infection dominate. Further along, BK virus, human papilloma virus, CMV, and
EBV-associated lympho- proliferative disease can appear.
 |
| Plate 10-28 CAUSES OF GRAFT DYSFUNCTION IN IMMEDIATE POST-TRANSPLANT PERIOD |
POSTOPERATIVE
PERIOD
Several
complications, both surgical and immunologic, may cause delayed graft function
(DGF) or failure of a previously functional graft. Therefore, it is essential
that patients undergo regular monitoring with measurement of serum creatinine
concentration. At some centers,
surveillance biopsies are also performed either on a routine basis or in select patients at high risk
for rejection.
The most
probable causes of graft dysfunction depend on the amount of time that has
passed since the transplantation.
Immediate
Postoperative Period. After a live donor transplant, the kidney begins
functioning right away in roughly 95% of cases. After a deceased donor trans-
plant, however, there may be some degree of DGF, which may last for days,
weeks, or even months.
Hyperacute
rejection occurs minutes to hours after transplantation, and it is often
diagnosed in the operating room immediately after revascularization of the
allograft. Such rejection reflects the presence of preformed antibodies that
target antigens on the allograft, such as HLA class I proteins, HLA class II
proteins, or ABO blood group antigens. A patient may become HLA-sensitized by
previous blood transfusions, pregnancies, or prior transplants. No matter the
cause, the presence of preformed antibodies leads to rapid immune complex formation, complement-mediated inflammation, and
activation of the coagulation cascade with subsequent allograft thrombosis. The
allograft is rapidly lost and must be removed. This complication is rarely seen
in current transplantation due to preoperative crossmatch testing performed
between the recipient serum and donor cells, as described previously.
In the
immediate postoperative period, acute tubular necrosis (ATN) is the most
frequent cause of DGF. Risk factors include prolonged cold ischemia times and
older age of the donor. Patients with ATN are offered supportive care because
spontaneous resolution after 1 or more weeks is common. ATN is a diagnosis of
exclusion, however, and thus other potential causes of DGF must be ruled out,
including prerenal state, thrombosis of the renal vessels, anatomic or
functional obstruction of the urine collecting system, and urine leak.
Prerenal
state, in which there is inadequate perfusion pressure in the allograft, may
occur secondary to volume depletion or vasodilation. To avert this
complication, patients should receive several liters of fluid in the operating
room, which will help offset the vasodilation associated with anesthesia. If
DGF occurs, the response to further fluid resuscitation should be included as
part of the routine evaluation. Rarely, patients may experience volume
depletion secondary to hemorrhage, possibly of the vascular anastomoses. If
major postoperative bleeding is suspected, immediate surgical reexploration
should be performed.
 |
| Plate 10-29 CAUSES OF GRAFT DYSFUNCTION IN EARLY POST-TRANSPLANT PERIOD |
Primary
thromboses of the renal vessels (i.e., not secondary to rejection) may occur
secondary to surgical technique or, more often, a hypercoagulable state (e.g.,
antiphospholipid antibody). Both arterial and venous thromboses may cause
sudden anuria; venous thromboses are also associated with pain around the
allograft. Both kinds of thromboses can only be treated with immediate surgical
reexploration, which usually reveals an infarcted graft that must be removed.
Thromboses can be diagnosed with color Doppler ultrasound, which will reveal an
absence of arterial and/or venous flow.
Obstruction
of the urinary collecting system is another possible cause of delayed graft
function. There are numerous possible causes, including benign prostatic hypertrophy,
neurogenic bladder, blood clots in the ureter, tight ureterovesical
anastomosis, and mal-positioning/obstruction of a urinary catheter.
Finally,
urine leak may present similarly to delayed renal function because it causes
low urine output and, as a result of urine reabsorption, an elevation in serum
creatinine and urea concentrations. Possible causes include ureteral infarction
or failure of the ureterovesical anastomosis. Urine leaks can usually be
diagnosed using ultrasonography or isotope renography. Stenting across the defect may be
attempted, although surgical reconstruction may be necessary in some cases.
Early
Post-transplant Period (1 Week to 6 Months). Many of the pathologies
that cause DGF may also cause renal dysfunction in the early post-transplant
period. Prerenal state, for example, may occur secondary to inadequate fluid
intake, diarrhea, or use of drugs that impair tubuloglomerular feedback, such
as ACE inhibitors or NSAIDs. In addition, renal vessel thromboses may not occur
until several weeks post-transplantation.
Postrenal disease, such as urinary tract
obstructions and urine leaks, may also occur during this period. In addition to
the obstructions that may be seen immediately following the transplantation,
patients may also develop lymphoceles, which occur secondary to disruption of
lymphatic channels around the iliac arteries. Lymphoceles can cause thigh
swelling and urinary frequency secondary to bladder compression. If very large,
they may compress the allograft ureter and cause renal dysfunction. The
diagnosis can be established using ultrasound or, as needed, analysis of a fluid
aspirate. Symptomatic lymphoceles may be treated with a combination of
ultrasound-guided drainage, followed by injection of sclerosing agents or, as
needed, surgical marsupialization.
The other
causes of early post-transplant graft dysfunction include various types of
intrarenal disease, including acute allograft rejection (either cellular or
antibody-mediated), calcineurin inhibitor nephrotoxicity, medication or
contrast-associated nephrotoxic ATN, acute pyelonephritis, and recurrence of
the primary renal disease.
 |
| Plate 10-30 PATHOLOGIC FINDINGS IN ACUTE REJECTION OF KIDNEY ALLOGRAFT |
Acute
allograft rejection can be either cellular or antibody-mediated. It is the most
frequent type of rejection, occurring in 10% to 15% of patients during the first
year after transplant. Manifestations include a rapid loss in renal function,
sometimes accompanied by low-grade fever and pain over the graft. More systemic
signs of illness, such as nausea or myalgias, have become uncommon with the use
of modern immunosuppression regimens. Acute rejection may occur as little as 1
week after transplantation but is typically seen after 1 to 3 months. It should
be strongly suspected in a patient with declining renal function but reasonable
plasma calcineurin inhibitor levels and no evidence of recurrent primary
disease (i.e., no proteinuria or evidence of glomerular bleeding). Because the
treatment strategies for cellular and antibody-mediated acute rejection are
different, a renal biopsy is essential for making the distinction.
Acute
cellular rejection results from an interaction between recipient
antigen-presenting cells (APCs), recipient T cells, and MHC antigens on donor
cells. The T cells become activated, resulting in the transcription of genes
for cytokines and cytokine receptors, leading to inflammation in the allograft.
Histopathologic findings include interstitial inflammation, predominantly by T
lymphocytes, accompanied by tubulitis, which occurs when T cells cross tubular
basement membranes and infiltrate tubular epithelium. Inflammation of arteries
(endarteritis) may also be noted. It usually begins as endotheliitis,
characterized by swelling and detachment of endothelial cells, as well as
lymphocyte infiltration of the endothelial layer. In severe cases, transmural
vasculitis may occur, in which lymphocytes infiltrate and inflame the entire
thickness of the vessel wall. Acute cellular rejection can usually be treated
with a pulse of high-dose corticosteroids or, in cases of steroid resistance,
antilymphocyte antibodies.
Acute
antibody-mediated rejection is less common than acute cellular rejection, and
it may result from a previous exposure to a specific antigen, or from de novo
reactivity and clonal expansion of reactive B cells. It typically occurs within
2 weeks of transplantation, and the presentation is similar to acute cellular rejection. Patients are
found to have antibodies that target donor HLA or ABO-group antigens.
Histopathologic findings can range from a subtle form of tubular injury, similar
to what is seen in ATN, to dramatic occlusion of glomerular capillaries by
neutrophils and fibrin-rich thrombi. One of the most common histologic
manifestations of acute antibody-mediated rejection is peritubular
capillaritis, characterized by dilation of the interstitial capillaries and
margination of leukocytes, most often a combination of neutrophils and
lymphocytes. A helpful marker of acute antibody-mediated rejection is the
presence of C4d within peritubular capillaries. C4d is a degradation product of
complement factor C4 and can be detected using either immunofluorescence or
immunohistochemistry. Acute antibody-mediated rejection can be treated with
plasmapheresis to remove the antibodies and infusion of intravenous immunoglobulin.
 |
| Plate 10-31 HISTOPATHOLOGIC FINDINGS IN CALCINEURIN INHIBITOR NEPHROTOXICITY |
Calcineurin
inhibitor (CNI) nephrotoxicity may also occur in the early postoperative
period, often secondary to drug-induced constriction of afferent arterioles.
The diagnosis should be suspected in a patient with a supra- therapeutic serum
calcineurin inhibitor concentration in whom renal function improves after dose
reduction. A lack of response to dose reduction, however, does not necessarily
exclude CNI-related disease. Thus a biopsy is often required to make the
distinction from acute rejection. If no significant pathologic changes are seen
at biopsy, the CNI toxicity is assumed to be a predominantly hemodynamic
effect. Several pathologic changes, however, are sometimes seen. CNI toxicity
can affect tubules, where it causes isometric vacuolization of the epithelial
cytoplasm. CNI toxicity can also affect vessels, where it causes hyalinosis of
medial myocytes. These changes are best appreciated in afferent arterioles.
Rarely, CNI toxicity can cause severe endothelial cell damage that results in
thrombotic microangiopathy, characterized by fibrin thrombus formation in small
arterioles and glomerular capillaries.
Numerous
medications may cause nephrotoxic ATN in allografts, as they do in native
kidneys (see Plate 4-3). Certain drugs, such as erythromycin, are especially nephrotoxic when
administered along with calcineuinhibitors because of their effects on hepatic
metabolism.
Pyelonephritis
may occur secondary to immunosuppression and frequent catheterization. Like
acute rejection, it may present as fever and allograft pain. Urine dipstick and
culture should be performed to assess for the presence of this complication.
Recurrence
of a primary renal disease, such as focal segmental glomerulosclerosis, may
also occur. Although glomerular
disease may sometimes be distinguished from rejection based on the presence of
heavy proteinuria or glomerular bleeding (i.e., red blood cell casts or
dysmorphic red blood cells) on urine sediment, biopsy is often required to make
the distinction.
Late
Post-Transplant Period (After 6 Months). Many of the problems that can occur in the
early post-transplant period may also occur in the late post- transplant
period, including prerenal state, CNI nephrotoxicity, nephrotoxic ATN, pyelonephritis, recurrence of primary
disease, and urinary tract obstruction. Late acute rejection may also occur in
patients with inadequate immunosuppression or medication non-compliance. The
other causes of late post-transplant graft dysfunction include chronic
allograft nephropathy, BK virus infection, and renal artery stenosis.
Chronic
allograft nephropathy, the most important cause of late allograft loss, is a
poorly characterized phenomenon. Most pathologists use the term to encompass a
myriad of structural and functional alterations related to chronic rejection
that develop over the course of months and generally cause loss of the graft
over a period of years. The major histologic findings include interstitial
fibrosis, tubular atrophy, chronic arterial and arteriolar inflammation with
luminal narrowing, and transplant glomerulopathy (which features doubling of
the glomerular basement membrane, as in membranoproliferative
glomerulonephritis).
BK virus is
a polyomavirus that infects many adults but only appears to cause disease in
those who are immunosuppressed. It has a particular tropism for the urinary
tract, where it can cause interstitial nephritis or ureteral stenosis. Urine
microscopy reveals “decoy cells,” which are tubular epithelial and urothelial
cells infected with the BK virus. Since anti-BK antibodies are found in many
individuals without BK-related disease, polymerase chain reaction (PCR) testing
is performed to detect the virus itself in urine and blood, sometimes on a
screening basis. A renal biopsy is performed if PCR is positive in the setting
of renal dysfunction. Characteristic histopathologic findings include
intranuclear inclusions within tubular epithelial cells, tubular injury,
tubulitis, and interstitial inflammation. Anti-SV40 immunohistochemistry is
performed to confirm the presence of viral antigen. Treatment usually consists
of reducing the dosage of immunosuppressive therapies.
 |
| Plate 10-32 CAUSES OF GRAFT DYSFUNCTION IN LATE POST-TRANSPLANT PERIOD IN KIDNEY TRANSPLATION |
Renal artery
stenosis (see Plate 4-36) may occur secondary to disease in either the donor or
recipient vasculature. Possible causes include vascular trauma and
atherosclerosis. Suggestive clinical features include hypertension, renal
dysfunction that is worsened upon provision of ACE inhibitors, weakened femoral pulses, and a new bruit
over the allograft. Percutaneous transluminal angioplasty may be required for
severe cases (see Plate 10-17).
Prognosis.
Despite
the risks associated with the transplantation procedure and allograft
rejection, the overall prognosis for patients who receive renal transplants is
excellent. The graft survival rates for deceased donor kidneys are 89%, 78%,
and 67% at 1, 3, and 5 years,
respectively; meanwhile, the graft survival rates for living donor kidneys are
95%, 88%, and 80% at 1, 3, and
5 years, respectively.
Because of
these positive outcomes, kidney trans- plantation is becoming widely practiced
around the world. Improvements in organ access, donation, preservation
techniques, immunosuppression, and management of disease progression will
further improve outcomes and access to transplantation in the future.




