SARCOIDOSIS
Sarcoidosis is a common disease of unknown origin characterized by the infiltration of many organs by non-caseating epithelioid granulomas. The lung is the most common organ affected by sarcoidosis. The skin, eye, and liver are also frequently involved. Sarcoidosis may affect many other organs, many of which are detailed in the next paragraph. Although in the United States sarcoidosis is most common in African Americans, the disease is also has a high prevalence in Northern Europeans and occurs worldwide. Women appear to contract the disease more often than men. The majority of patients are younger than 40 years of age at onset, although there is a second peak of increased incidence after age 50 years in women. There is a higher incidence of the disease in first-degree relatives (parents, siblings, and children) of sarcoidosis patients than the general population. This is in keeping with the belief that sarcoidosis represents an abnormal granulomatous response to an environmental exposure in genetically susceptible individuals. Sarcoidosis is rare in people younger than age 18 years. Sarcoidosis is often a benign condition that may run its entire course without detection. It is often discovered in asymptomatic patients on screening chest radiographs.
Sarcoidosis may present as a variety of clinical
syndromes, which vary primarily depending on the distribution of granulomatous
involvement of the affected organs (see Plate 4-155). These include (1) Löfgren
syndrome (erythema nodosum with radiographic evidence of hilar lymph node
enlargement, often with concomitant fever and joint [often ankle] arthritis);
(2) cutaneous plaques and subcutaneous nodules; (3) Heer fordt syndrome (uveoparotid fever);
(4) isolated uveitis; (5) salivary
gland enlargement; (6) central nervous system (CNS) syndromes (usually seventh
nerve palsy); (7)
cardiomyopathy or cardiac arrhythmias; (8) hepatosplenomegaly (with or without
hypersplenism); (9) upper airway involvement (sarcoidosis of the upper
respiratory tract [SURT]); (10) hypercalcemia; (11) renal failure; (12)
peripheral lymphadenopathy; and (13) various forms of pulmonary disease,
including mediastinal adenopathy, interstitial lung disease, endobronchial
involvement with airflow obstruction and wheezing, and pulmonary hypertension.
 |
| Plate 4-155 |
Pulmonary hypertension is a potentially
life-threatening complication of sarcoidosis. Sarcoidosis associated pulmonary
hypertension is classified in the miscellaneous category (class 5) according to
the World Health Organization classification scheme. This is because there are
multiple mechanisms that may cause pulmonary hypertension in sarcoidosis,
including pulmonary venous hypertension from myocardial involvement, pulmonary
fibrosis causing vascular distortion, hypoxemia from parenchymal sarcoidosis,
compression of the vasculature from the thoracic lymphadenopathy of
sarcoidosis, and direct granulomatous involvement of the pulmonary vasculature.
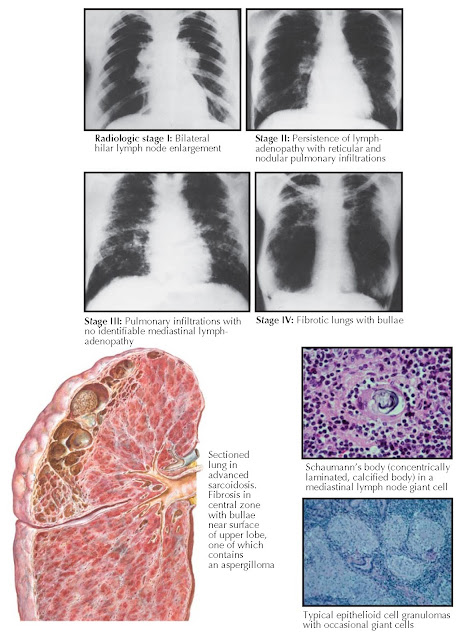
The radiographic presentations of sarcoidosis have
been divided into five stages: (0) a normal chest radiograph, (I) bilateral
hilar and right paratracheal lymph node enlargement, (II) persistence of lymph
nodes with concomitant pulmonary infiltrations, (III) pulmonary infiltrations
with no identifiable mediastinal adenopathy, and (IV) fibrocystic changes that are
usually most prominent in the upper lobes. The fibrosis may be significant, with
retraction of the hilar areas upward and unilateral deviation of the trachea.
Occasionally, aspergillomas may develop in these fibrocystic spaces. Patients
with radiographic stage I sarcoidosis are most often asymptomatic and usually
have normal pulmonary function test results despite the universal presence of
granulomas on lung biopsy specimens at this stage of the disease. With
radiographically discernible pulmonary lesions, a restrictive pattern of
dysfunction may emerge, with loss of lung volumes; decreased pulmonary
compliance; hyperventilation; decreased diffusing capacity; and in the most
severely afflicted patients, hypoxemia. In chronically scarred lungs, evidence
of airway dysfunction usually appears, with decreased FEV1 (forced expiratory volume in
1 second) and diminished flow rates at low lung volumes. Although dyspnea,
pulmonary dysfunction, and prognosis are generally worse with higher
radiographic stages, there is too much overlap for this to be useful to assess
individual patients. It is clear that patients with stage IV radiographs
include nearly all the patients with a very poor prognosis, although not all
patients with stage IV radiographs will fare poorly.
Although chest computed tomography scanning is not
required to assess the status of pulmonary sarcoidosis, it often clearly
identifies mediastinal adenopathy. Furthermore, it may detect parenchymal
disease that is not evident on chest radiographs. Parenchymal sarcoidosis is
commonly located along the bronchovascular bundles and in subpleural locations.
Noncaseating epithelioid granulomas, often accompanied
by giant cells and rarely by small, calcified bodies (Schaumann bodies), are the
fundamental pathologic lesions in sarcoidosis but are nonspecific (see Plate
4-156). However, these granulomas often
cannot be differentiated from the granulomas of fungal infections, berylliosis,
leprosy, brucellosis, hypersensitivity lung diseases, the occasional instances
of tuberculosis when caseation and acid-fast bacilli are not apparent, and
lymph nodes draining neoplastic tumors. Therefore, the diagnosis of sarcoidosis
requires a compatible clinical picture and negative smears and cultures for
organisms causing the diseases. Granulomas frequently develop in several
organs, accounting for the multiple modes of clinical presentation when organ
structure and function are impaired. In the majority of patients with
disability, the organs primarily affected are the lungs, eyes, and myocardium.
The immunopathogenesis of sarcoidosis is not
completely understood. The process probably begins with the interaction of
unknown antigen(s) with antigen-presenting cells (APCs) such as dendritic cells
and macrophages. It is postulated that these APCs process these antigens and
present them via human leukocyte antigen class II molecules to T-cell receptors
attached to T lymphocytes, usually of the CD4+ class. After these events occur, T
cells are stimulated to proliferate, and cytokines including interleukin-2 and
interferon-, are produced. These cytokines are thought to enhance pro- duction
of macrophage-derived tumor necrosis factor-(TNF-α). These cytokines and undoubtedly many others are
responsible for granuloma formation.
Elevated levels of serum angiotensin-converting enzyme (ACE) have been observed in
active sarcoidosis. However, the serum ACE level is thought not to be specific
or sensitive enough for the diagnosis of sarcoidosis. The serum ACE level may
be useful to measure disease activity in cases in which clinical methods of
assessment are difficult or costly.
The diagnosis of sarcoidosis rests on the
demonstration of noncaseating epithelioid granulomas in tissues subjected to
biopsy (skin, lymph nodes, or lung) from a patient with a compatible clinical
picture. As previously mentioned, the clinician must be vigilant that alternate
potential causes of granulomatous inflammation have been reasonably excluded.
 |
| Plate 4-156 |
The majority of patients with sarcoidosis can expect a
benign course with complete clearing or nondisabling persistence of
radiographic and other clinical abnormalities. However, a small but significant
number of patients will be disabled, and approximately 4% will die of their
sarcoidosis, usually from respiratory failure. Less commonly, death occurs from
sarcoid cardiomyopathy or CNS involvement. For unknown reasons, cardiac
involvement is the major cause of death from sarcoidosis in Japanese
individuals. Rarely, death may be the result of renal failure or from
hemorrhage because of pulmonary aspergillomas that form in sarcoid bullae.
African Americans tend to have more aggressive forms of sarcoidosis than
whites.
Patients with active sarcoidosis usually respond well
to corticosteroids. The usual course of therapy for acute pulmonary sarcoidosis
is 20 to 40 mg/d prednisone equivalent for 6 to 12 months. Relapse is common
after cessation of prednisone and may require reinstitution of treatment.
Higher doses of corticosteroids
are often required for cardiac involvement, disfiguring facial sarcoidosis (lupus
pernio), and neurosarcoidosis. Prompt treatment with corticosteroids is
indicated for patients with uveitis, CNS disease, hypercalcemia,
cardiomyopathy, hypersplenism, and progressive pulmonary dysfunction, but only
10% of patients with sarcoidosis require mandatory treatment of this kind.
Corticosteroids are not indicated in patients with asymptomatic hilar
lymphadenopathy or minor radiographic pulmonary shadows or for asymptomatic
elevations in serum liver function tests. The arthritis of Löfgren syndrome can usually be managed with nonsteroidal
antiinflammatory agents.
Because prolonged corticosteroid therapy is hazardous,
alternative medications to corticosteroids are often used for chronic
sarcoidosis. In these instances, corticosteroids are often still required, but
the addition of alternative medicines has a corticosteroid-sparing effect such
that the maintenance corticosteroid dose can be reduced. Such medications
include methotrexate, hydroxychloroquine, chloroquine, azathioprine,
leflunomide, pentoxifylline, thalidomide, the tetracyclines, and infliximab.




