PULMONARY PHARMACOLOGY
 |
| BRONCHODILATORS |
Pulmonary pharmacology concerns the effects of drugs on the lungs and understanding how drugs used to treat patients with pulmonary diseases work. Much of this pharmacology concerns drugs used to treat obstructive airway diseases, such as asthma and chronic obstructive pulmonary disease (COPD).
Two types of drugs are used in the treatment of
obstructive airway diseases:
1. Relievers (bronchodilators) give immediate reversal of
airway obstruction, largely by directly relaxing airway smooth muscle.
2. Controllers (preventers) suppress the underlying
disease process and provide long-term control of symptoms. These drugs include
anti inflammatory treatments, such as
corticosteroids.
Both asthma and COPD are characterized by airway
narrowing secondary to a chronic inflammatory process. In asthma, eosinophilic
(and sometimes neutrophilic) inflammation occurs throughout the respiratory
tract, although the proximal airways are predominantly affected. In COPD, there
is inflammation and narrowing of small airways (chronic obstructive
bronchiolitis) and destruction of lung parenchyma (emphysema), resulting in
loss of support for the airways, early closure on expiration, and air trapping.
Bronchodilators cause immediate reversal of airway
obstruction as a result of a relaxing effect on airway smooth muscle. However,
other pharmacologic effects of bronchodilator drugs on other airway cells
(reduced microvascular leakage, reduced release of bronchoconstrictor mediators
from inflammatory cells) may contribute to the reduction in airway narrowing.
Three classes of bronchodilators are in current clinical use for the treatment
of obstructive airway diseases: β2- agonists,
theophylline, and anticholinergics.
2-ADRENERGIC AGONISTS
Inhaled β2-agonists are
the bronchodilator treatment of choice for patients with asthma because they
are the most effective bronchodilators, reverse all known bronchoconstrictor
mechanisms, and have minimal side effects when used correctly. Short-acting and
nonselectiveagonists (e.g., isoproterenol) have no role.
Mode of Action
β2-Agonists
produce bronchodilatation by directly stimulating β2-receptors on airway smooth muscle cells, which leads
to relaxation of central and peripheral airways. β2-agonists act as “functional antagonists” and reverse
bronchoconstriction irrespective of the contractile agent; this is important in
asthma because many bronchoconstrictor mechanisms (neural and mediators) are
likely to constrict airways. In COPD, their major effect is reversal of
cholinergic neural tone. Occupation of β2-receptors by
agonists results in the activation of adenylyl cyclase via the stimulatory G-protein (Gs),
which increases intracellular cyclic AMP (cAMP), leading to relaxation through
inhibition of the contractile machinery.
β2-receptors are
localized to several types of airway cells, and β2-agonists may
have additional effects.β2-agonists may
cause bronchodilatation, not only by a direct action on airway smooth muscle
but also indirectly by inhibiting the release of bronchoconstrictor mediators from mast cells and of
bronchoconstrictor neurotransmitters from airway nerves. β2-agonists have an inhibitory effect on mast cell
mediator release and microvascular leakage, suggesting they may inhibit acute
inflammation. However, β2-agonists do
not have a significant inhibitory effect on the chronic inflammation of asthmatic
airways and do not reduce airway hyperresponsiveness, which is a clinical
manifestation of inflammation
in asthma.
Short-acting inhaled β2-agonists (e.g., albuterol, terbutaline) are the most widely
used bronchodilators. Their duration of action is 3 to 4 hours (less in severe
asthma). When inhaled from pressurized metered dose inhalers (pMDIs) in
standard doses, they are convenient, easy to use, rapid in onset, and without
significant side effects. They also protect against bronchoconstrictor stimuli
such as exercise, cold air, and allergens. They are the bronchodilators of
choice in acute severe asthma, in which the nebulized route of administration is
as effective as intravenous use. The inhaled route of administration is
preferable to the oral route because side effects are less common and because
it may be more effective (better access to surface cells such as mast cells).
Short-acting inhaled β2-agonists should be used as required by symptoms and
not on a regular basis; increased usage indicates a need for more
antiinflammatory therapy.
Long-Acting Inhaled 2-Agonists
The long-acting inhaled β2-agonists
(LABAs) salmeterol and formoterol are a significant advance in the treatment of
patients with asthma and COPD. Both drugs have a bronchodilator action, protect
against bronchoconstriction for more than 12 hours, and provide better symptom
control (when given twice daily) than regular treatment with short-acting β2- agonists
(four times daily). Formoterol has a more rapid onset of action but is a fuller
agonist than salmeterol, so tolerance is more likely. Inhaled long-actingβ2-agonists may
be added to low or moderate doses of inhaled corticosteroids if asthma is not
controlled, and this is more effective than increasing the dose of inhaled
corticosteroids. Long-acting inhaled β2-agonists should be used only in patients who are
taking inhaled corticosteroids because these drugs do not have an antiinflammatory
action and are potentially dangerous without corticosteroids. Combination
inhalers with a long-acting β2-agonist and corticosteroid (fluticasone/ salmeterol,
and budesonide/formoterol) are an effective and convenient way to control
asthma and are useful in COPD.
Side Effects
Unwanted effects result from stimulation of
extrapulmonary β-receptors and include tachycardia, tremors, and
palpitations. Side effects are uncommon with inhaled therapy but more common
with oral or intravenous administration.
Long-Term Safety
A large trial in the United States showed that
salmeterol increased mortality in patients with asthma, but this was mainly in
patients who were not using concomitant inhaled corticosteroids. This provides
a strong argument for only prescribing
long-actingβ2-agonists in a combination inhaler.
Tolerance
Continuous treatment with an agonist often leads to
tolerance (desensitization), which may result from uncoupling or downregulation
(or both) of the receptor. Tolerance of non-airway β-receptor responses (e.g., tremor, cardiovascular and
metabolic responses) is readily observed. Loss of bronchodilator action is minimal, but there is some loss of
bronchoprotective effect against, for example, exercise. This is incomplete and
not progressive and does not appear to be a clinical problem.
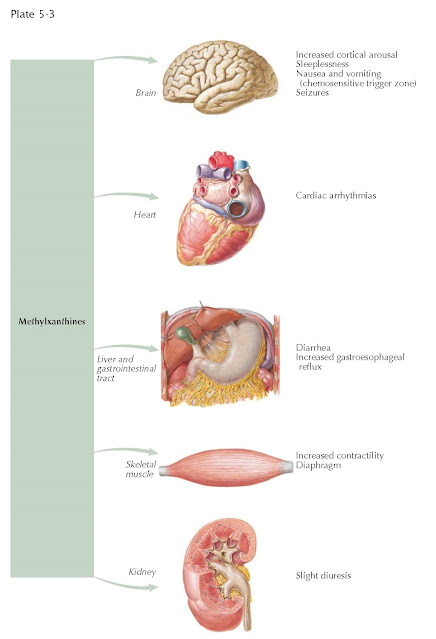
THEOPHYLLINE (METHYLXANTHINES)
Worldwide, theophylline remains the most widely used antiasthma therapy because it is inexpensive, but the greater incidence of side effects with theophylline and the greater efficacy of 2-agonists and inhaled corticosteroids have reduced its use (see Plate 5-2). It still remains a useful drug in patients with severe asthma and COPD. There is increasing evidence that low-dose theophylline (plasma concentration, 5-10 mg/L) has an anti inflammatory or immunomodulatory effect and may be effective in combination with inhaled corticosteroids.
Mode of Action
Despite extensive study, it has been difficult to
elucidate the molecular mechanisms of the antiasthma actions of theophylline.
It is possible that any beneficial effect in asthma is related to its action on
other cells (e.g., plate- lets, T lymphocytes, macrophages) or on airway micro-
vascular leak and edema in addition to airway smooth muscle relaxation.
Theophylline is a relatively ineffective bronchodilator, and high doses are
needed for its bronchodilator action. Its antiasthma effect is more likely to
be explained by other effects (e.g., immunomodulation). Several molecular modes
of action have been proposed.
Inhibition of Phosphodiesterases
Phosphodiesterases (PDEs) break down cAMP in the cell;
their inhibition leads to an increase in intracellular cAMP concentrations (see
Plate 5-2). PDE inhibition is likely to account for the bronchodilator action
of theophylline, but the degree of inhibition is relatively small at
concentrations of theophylline within the therapeutic range. PDE inhibition
also accounts for the side effects of nausea and headaches.
Adenosine Receptor Antagonism
Adenosine is a bronchoconstrictor in asthmatic
patients via activation of mast cells (A2B receptors). Adenosine
antagonism may account for some side effects of theophylline (e.g., central
nervous system [CNS] stimulation, cardiac arrhythmias, diuresis).
Histone Deacetylase Activation
Therapeutic concentrations of theophylline activate
histone deacetylases in the nucleus, resulting in the switching off of
inflammatory genes and enhancing the antiinflammatory action of corticosteroids,
especially when there is corticosteroid resistance.
 |
| METHYLXANTHINES: ADVERSE EFFECTS |
Clinical Use
In patients with acute asthma, intravenous
aminophylline is less effective than nebulized β2-agonists and should therefore be reserved for the few
patients who fail to respond to β-agonists.
(Aminophylline is a stable mixture or combination of theophylline and
ethylenediamine, which confers greater solubility.) Theophylline is less
effective as a bronchodilator than inhaled β2- agonists and is more likely to have side effects.
There is increasing evidence that low
doses (giving plasma concentrations
of 5-10 mg/L) may be useful when added to inhaled corticosteroids, particularly
in more severe asthma. Theophylline is also useful as an additional
bronchodilator in COPD, reducing hyperinflation and improving dyspnea.
Theophylline is readily and reliably absorbed from the
gastrointestinal tract, but many factors affect plasma clearance, and thereby
plasma concentration, that make the drug relatively difficult to use.
Side Effects
Adverse effects are usually related to plasma
concentration and tend to occur when plasma levels exceed 20 mg/L, although
some patients develop them at lower plasma concentrations. The severity of side
effects may be reduced by gradually increasing the dose until therapeutic
concentrations are achieved. The most common side effects are headache, nausea
and vomiting, abdominal discomfort, and restlessness, which are likely caused by PDE inhibition and at
higher concentrations cardiac arrhythmias and seizures caused by antagonists of
adenosine A1-receptors. Theophylline also has many interactions with other
drugs because of alterations in liver enzyme metabolism.
ANTICHOLINERGICS
Atropine is a naturally occurring compound that was
introduced for the treatment of asthma but because of side effects
(particularly drying of secretions), less soluble quaternary compounds (e.g.,
ipratropium bromide) were developed.
Mode of Action
Anticholinergics are specific antagonists of muscarinic receptors and inhibit cholinergic nerve-induced bronchoconstriction. A small degree of resting bronchomotor tone is present because of tonic cholinergic nerve impulses, which release acetylcholine in the vicinity of airway smooth muscle, and cholinergic reflex bronchoconstriction may be initiated by irritants, cold air, and stress. Although anticholinergics protect against acute challenge by sulfur dioxide and emotional factors, they are less effective against antigen, exercise, and fog; they inhibit reflex cholinergic bronchoconstriction only and have no significant blocking effect on the direct effects of inflammatory mediators, such as histamine and leukotrienes. In COPD, cholinergic tone is the major reversible element of airway narrowing.
Clinical Use
Whereas ipratropium bromide and oxitropium bromide are
administered three or four times daily via inhalation, tiotropium bromide is
given once daily. In patients with asthma, anticholinergic drugs are less
effective than β2-agonists and offer less protection against various
bronchial challenges. Nebulized anticholinergics are effective in acute severe
asthma but less effective thanβ2-agonists. Nevertheless, anticholinergic drugs may
have an additive effect with β2-agonists in acute and chronic treatment and should
therefore be considered when control of asthma is inadequate, particularly when
there are side effects with theophylline or inhaled β-agonists.
Anticholinergic drugs are the bronchodilators of
choice in COPD, and once-daily tiotropium bromide is the most effective
bronchodilator for COPD.
Side Effects
Inhaled anticholinergic drugs are well tolerated, and
systemic side effects are uncommon because almost no systemic absorption occurs.
Ipratropium bromide, even in high doses, has no detectable effect on airway
secretions. Nebulized ipratropium bromide may precipitate glaucoma in elderly patients as a
result of a direct effect of the
nebulized drug on the eye; this is avoided by use of a mouthpiece rather than a
face mask. Paradoxic bronchoconstriction with ipratropium bromide, particularly
when given by nebulizer, was largely explained by the hypotonicity of an
earlier nebulizer solution and by antibacterial additives such as benzalkonium
chloride; this problem is avoided with current preparations. Dry mouth occurs
in about 10% of patients taking tiotropium bromide but rarely requires discontinuation
of treatment.
CORTICOSTEROIDS
Corticosteroids are the most effective therapy
available for asthma (see Plates 5-5 and 5-6). Inhaled corticosteroids have
revolutionized the management of patients with chronic asthma and are now used as first-line
therapy in all patients with persistent symptoms.
Mode of Action
Corticosteroids enter target cells and bind to
glucocorticoid receptors in the cytoplasm. The corticosteroidreceptor complex
is transported to the nucleus, where it binds to specific sequences on the
upstream regulatory element of certain target genes, resulting in increased or
decreased transcription of the gene and increased or decreased protein
synthesis. Glucocorticoid receptors may also inhibit transcription factors,
such as nuclear factor-ƘB and activator
protein-1, which regulate inflammatory gene expression by a nongenomic
mechanism. Corticosteroids inhibit acetylation of core histones and thereby
inflammatory gene expression by recruiting histone deacetylase-2 to the
activated transcriptional complex.
The mechanism of action of corticosteroids in asthma
is most likely related to their antiinflammatory properties. Corticosteroids
have widespread effects on gene transcription, increasing transcription of
antiinflammatory genes and more importantly suppressing transcription of
multiple inflammatory genes. At a cellular level, they have inhibitory effects
on many inflammatory and structural cells that are activated in asthma. The
inhibitory action of inhaled corticosteroids on airway epithelial cells may be
particularly important; this results in a reduction in airway hyperresponsiveness,
but in long-standing asthma, airway hyperresponsiveness may not return to
normal because of irreversible structural changes in airways.
Clinical Use
Systemic corticosteroids are used in acute asthma and
accelerate its resolution. There is no advantage with very high doses of
intravenous corticosteroids (e.g., methylprednisolone, 1 g). Prednisolone or
prednisone (40-60 mg orally) has an effect similar to intravenous
hydrocortisone and is easier to administer.
Maintenance doses of oral corticosteroids are reserved
for patients whose asthma cannot be controlled on other therapy; the dose is
titrated to the lowest that provides acceptable symptom control. In any patient
taking regular oral corticosteroids, objective evidence of corticosteroid responsiveness
should be obtained before maintenance therapy is instituted. Short courses of
oral corticosteroids (prednisolone, 30-40 mg/d for 1-2 weeks) are indicated for
exacerbations of asthma; the dose may be tapered over 1 week after the
exacerbation is resolved. (The tapering period is not strictly necessary, but
patients find it reassuring.) Inhaled corticosteroids are currently recommended
as first-line therapy in all patients with persistent asthma. Inhaled
corticosteroids, such as beclomethasone dipropionate, budesonide, fluticasone
propionate, triamcinolone, mometasone furoate, and ciclesonide, act topically
on the inflammation in the airways of asthmatic patients. They may be started in
any patient who needs to use a β2-agonist inhaler for symptom control more than twice a
week. In most patients, inhaled
corticosteroids are used twice daily; this improves compliance after control of asthma has been
achieved. If a dose of more than 800 µg of budesonide or equivalent daily via
MDI is administered, a spacer should be used to reduce the risk of
oropharyngeal side effects and of absorption from the gastrointestinal tract.
Inhaled corticosteroids at doses of 400 µg/d or less may be used safely in
children.
Rarely, patients with severe asthma fail to respond to
corticosteroids. Corticosteroid-resistant asthma is likely to be caused by several
molecular mechanisms, including defective translocation of the glucocorticoid
receptor as a result of activated kinases or reduced histone deacetylase-2
activity. COPD patients occasionally respond well to corticosteroids; these
patients are likely to have undiagnosed asthma. Patients with COPD show a poor
response to corticosteroids, and the inflammation is essentially steroid
resistant. The steroid resistance in COPD appears to be caused by a marked reduction in
histone deacetylase-2 in inflammatory cells, such as macrophages. Inhaled
corticosteroids have no effect on the progression of COPD but reduce
exacerbations in patients who have severe disease and frequent exacerbations.
Inhaled corticosteroids do not reduce mortality in COPD, and recent evidence
suggests that in high doses, they may increase the risk of developing
pneumonia.
Side Effects (see Plate 5-7)
Corticosteroids inhibit cortisol secretion by a
negative feedback effect on the pituitary gland. Hypothalamopituitary–adrenal
axis suppression is dependent on dose and usually occurs when a dose of
prednisone of more than 7.5-10 mg/d is used. Significant suppression after short
courses of corticosteroid therapy is not usually a problem, but prolonged
suppression may occur after several months or years; corticosteroid doses after
prolonged oral therapy must therefore be reduced slowly. Symptoms of
“corticosteroid withdrawal syndrome” include lassitude, musculoskeletal pains,
and occasionally fever.
Side effects of long-term oral corticosteroid therapy
include fluid retention, increased appetite, weight gain, osteoporosis,
capillary fragility, hypertension, peptic ulceration, diabetes, cataracts, and
psychosis. The incidence tends to increase with age.
Systemic side effects of inhaled corticosteroids have
been investigated extensively. Effects such as cataract formation and
osteoporosis are reported but often in patients who are also receiving oral
corticosteroids. There has been particular concern about growth suppression in
children using inhaled corticosteroids, but in most studies, doses of 400 µg
or less have not been associated with impaired growth, and there may even be a
growth spurt because asthma is better controlled. The fraction of
corticosteroid inhaled into the lungs acts locally on the airway mucosa and may
be absorbed from the airway and alveolar surface, thereby reaching the systemic
circulation. The fraction of inhaled corticosteroid deposited in the oropharynx
is swallowed and absorbed from the gut. The absorbed fraction may be
metabolized in the liver before it reaches the systemic circulation. Budesonide
and fluticasone propionate have a greater first-pass metabolism than
beclomethasone dipropionate and are therefore less likely to produce systemic
effects at high inhaled doses. The use of a large volume spacer reduces
oropharyngeal deposition, thereby reducing systemic absorption of
corticosteroid.
· Initial studies suggested that adrenal suppression occurred only when inhaled doses of
more than 1500 µg/d were used.
· More sensitive measurements of systemic effects
include indices of bone metabolism (e.g., serum osteocalcin, urinary pyridinium
cross-links), 24-hour plasma cortisol profiles and, in children, short-term
growth of the lower leg, which may be affected by inhaled doses as low as 800 µg.
The clinical relevance of these measurements is unclear. Nevertheless, it is
important to reduce the risk of systemic effects by using the lowest dose of
inhaled corticosteroid needed to control the
asthma and by use of a large-volume spacer to reduce oropharyngeal deposition.
Inhaled corticosteroids may have local side effects
caused by deposition of corticosteroid in the oropharynx. These side effects
include oral thrush caused by overgrowth of Candida spp., throat
irritation, and changes in voice caused by vocal cord irritation and weakness.

ADVERSE EFFECTS OF CORTICOSTEROIDS
CROMONES
Cromones include cromolyn sodium and the structurally
related nedocromil sodium.
Mode of Action
Initial investigations suggested that cromoglycate
acts as a mast cell stabilizer, but this effect is weak in human mast cells.
Cromones inhibit bronchoconstriction induced by sulfur dioxide, metabisulfite,
and bradykinin, which are believed to act through
activation of sensory nerves in the airways. Cromones have variable inhibitory
actions on other inflammatory cells that may participate in allergic
inflammation, including macrophages and eosinophils.
Cromoglycate blocks the early response to allergen
(mediated by mast cells) and the late response and airway hyperresponsiveness,
which are more likely to be mediated by macrophage and eosinophil interactions.
The molecular mechanism of cromone action is not understood; evidence suggests
they may block a type of chloride channel that may be expressed in sensory
nerves, mast cells, and other inflammatory cells.
Clinical Use
Cromones are prophylactic treatments and must be given
regularly. They protect against indirect bronchoconstrictor stimuli, such as
exercise, allergens, and fog. Cromones are poorly effective compared with low
doses of inhaled corticosteroids, and recent systematic reviews concluded that
they provide little benefit in chronic asthma in children. Cromones are
administered four times daily and may also be taken before exercise in children
with exercise-induced asthma. There has been an increasing tendency to
substitute low-dose inhaled corticosteroids for cromoglycate in adults and
children, so they are now rarely used and are not recommended in most
guidelines. There is no role for cromones in the management of patients with
COPD.
Side Effects
Cromoglycate is one of the safest drugs available, and
side effects are extremely rare. The dry-powder inhaler may cause throat irritation; coughing;
and, occasionally, wheezing, but this is usually prevented by prior
administration of a β-agonist inhaler. Very rarely, a transient rash and
urticaria or pulmonary eosinophilia are seen; these result from
hypersensitivity. Side effects are not usually a problem with nedocromil,
although some patients have noticed a sensation of flushing after using the
inhaler.
ANTILEUKOTRIENES
Antileukotrienes (leukotriene receptor antagonists)
are less effective than inhaled corticosteroids in the control of asthma but
have been widely used because they are effective by mouth and have few side
effects (see Plates 5-8 and 5-9).
Mode of Action
Elevated levels of leukotrienes are detectable in
bronchoalveolar lavage fluid, exhaled breath condensate, sputum, and urine of
asthmatic patients. Cysteinyl-leukotrienes (cys-LTs) are generated from
arachidonic acid by the rate-limiting enzyme 5-lipoxygenase. Cys-LTs are potent
constrictors of human airways in vitro and in vivo, cause airway microvascular
leakage in animals, and stimulate airway mucus
secretion. These effects are all mediated in human airways via cys-LT1
receptors. Montelukast and zafirlukast are potent cys-LT1 receptor
antagonists that markedly inhibit the bronchoconstrictor response to inhaled
leukotrienes; reduce allergen-induced, exercise-induced, and cold air–induced
asthma by about 50% to 70%; and inhibit aspirin-induced responses in
aspirin-sensitive asthmatics almost completely. The only 5-lipoxygenase inhibitor clinically available is
zileuton, the efficacy of which is similar to that of receptor antagonists.
Antileukotrienes have also been shown to have weak antiinflammatory effects and
reduce eosinophilic inflammation, which may be provoked by cys-LTs.
Clinical Use
Antileukotrienes may have a small and variable
bronchodilator effect, indicating that leukotrienes may contribute to
baseline bronchoconstriction in asthma. Long-term administration reduces asthma
symptoms and the need for rescue 2-agonists and improves lung function.
However, their effects are significantly less than those with low-dose inhaled
corticosteroids in terms of symptom control, improvement in lung function, and
reduction in exacerbations. Antileukotrienes are not as effective as inhaled
corticosteroids in the management of mild asthma and are not the preferred
therapy. They may be useful in some patients whose asthma is not controlled on
inhaled corticosteroids as an add-on therapy to inhaled corticosteroids but are
less effective in this respect than a long- acting β2-agonist or
low-dose theophylline. They are effective in some but not all patients with
aspirinsensitive asthma. Patients appear to differ in their response to
antileukotrienes, and it is impossible to predict which patients will respond
best even when genetic polymorphisms of the leukotriene pathways are
elucidated.
A major advantage of antileukotrienes is that they are
orally active, and this is likely to improve compliance with long-term therapy.
However, they are expensive, and a trial of therapy is indicated to determine
which patients will benefit most.
Side Effects
Adverse effects are uncommon. Zafirlukast may produce
mild hepatic dysfunction, so regular liver function tests are important.
Several cases of Churg-Strauss syndrome (systemic vasculitis with eosinophilia
and asthma) have been observed in patients taking antileukotrienes, but this is
likely to be because a concomitant reduction in oral corticosteroids (made possible
by the antileukotriene) allows the vasculitis to flare up.
ANTI-IgE THERAPY
Mode of Action
Omalizumab is a humanized recombinant monoclonal
antibody that binds to circulating IgE and thus blocks it from activating
high-affinity IgE receptors on mast cells and low-affinity IgE receptors on other
inflammatory cells. This results in reduced responses to allergens. Over time,
the blocking of IgE reduces its synthesis from B cells and results in a
sustained reduction in IgE.
Clinical Use
Omalizumab reduces airway inflammation in patients with
mild to moderate asthma and reduces the incidence of asthma exacerbations with
improved control of asthma in patients maintained on reduced doses of inhaled
corticosteroids. Omalizumab is most useful in patients with severe asthma who
are not controlled with maximal doses of inhaled therapy because it reduces
exacerbations and improves asthma control. Fewer than 30% of patients show a
good response, and this is not
predictable by any clinical features; therefore, a trial of therapy over 4
months is indicated. Omalizumab should be given only to patients with serum IgE
levels of 20 to 700 IU/mL; above these levels, it is not possible to give
enough antibody to neutralize IgE. The dose of omalizumab is determined by the
serum IgE levels and is given either once or twice a month. Because of its high
cost only patients at steps 4
(severe) and 5 (very severe) of the Global Initiative for Asthma (GINA)
Guidelines who have frequent exacerbations are suitable for this therapy.
Side Effects
Omalizumab is well tolerated. Occasionally, local
reactions occur at the injection sites, and very rarely, anaphylactic reactions
have been seen.
IMMUNOSUPPRESSIVE AND CORTICOSTEROID-SPARING
THERAPY
Immunosuppressive therapy has been considered in
asthma when other treatments have been unsuccessful or when a reduction in the
dosage of oral corticosteroids is required; it is therefore indicated in very
few (1%) asthmatic patients at present.
Methotrexate
Low-dose methotrexate, 15 mg weekly, has a
corticosteroid-sparing effect in some patients with asthma, but side effects
are relatively common and include nausea (reduced if methotrexate is given as a
weekly injection), blood dyscrasia, hepatic damage, and pulmonary fibrosis.
Careful monitoring (monthly blood counts and liver enzymes) is essential.
Gold
Gold has long been used in the treatment of patients
with chronic arthritis. A controlled trial of an oral gold preparation
(auranofin) demonstrated some corticosteroid-sparing effect in chronic asthmatic
patients maintained on oral corticosteroids, but side effects (skin rashes and
nephropathy) are a limiting factor.
Cyclosporine A
Low-dose oral cyclosporine A in patients with
corticosteroid-dependent asthma is reported to improve control of symptoms, but
in clinical practice, it is unimpressive, and its use is limited by severe side
effects (nephrotoxicity, hypertension).
ANTITUSSIVES
Despite the fact that cough is a common symptom of
airway disease, its mechanisms are poorly understood, and current treatment in
unsatisfactory (see Plate 5-10). Because cough is a defensive reflex, its
suppression may be inappropriate in those with bacterial lung infections.
Before treatment with antitussives, it is important to identify underlying
causal mechanisms that may require therapy. Treatments such as opioids may act
centrally on the “cough center,” but other treatments such as local anesthetics
may act on airway sensory nerves.
Opiates have a central mechanism of action on the
medullary cough center, but some evidence suggests that they may have
additional peripheral action on cough receptors in the proximal airways.
Codeine and dextromethorphan are commonly used, but there is little evidence
that they are clinically effective. Morphine and methadone are effective but
are only indicated in patients with intractable cough associated with bronchial
carcinoma.
Asthma commonly presents as cough, and the cough
usually responds to bronchodilators and inhaled corticosteroids. A syndrome
characterized by cough in association with sputum eosinophilia but no airway
hyperresponsiveness and termed eosinophilic bronchitis responds to
inhaled corticosteroids and may be regarded as pre-asthma. Nonasthmatic cough
does not respond to inhaled steroids but sometimes responds to cromones or
anticholinergic therapy. The cough associated with postnasal drip of sinusitis
responds to antibiotics, nasal decongestants, and intranasal steroids. The
cough associated with angiotensin-converting enzyme inhibitors responds to
withdrawal of the drug (or a switch to
an angiotensin receptor antagonist) and to cromones. In some patients, there
may be underlying gastroesophageal reflux, which leads to cough by a reflex
mechanism and occasionally by acid aspiration. This cough responds to effective
suppression of gastric acid with an H2-receptor antagonist or more
effectively to a proton pump inhibitor, such as omeprazole.
Some patients have an intractable cough that often
starts after a severe respiratory tract infection. When no other causes for
this cough are found, it is termed idiopathic and may be caused by
hyperesthesia of airway sensory nerves. This is supported by the fact that
these patients have an increased responsiveness to tussive stimuli such as capsaicin.
This form of cough is difficult to manage. It may respond to nebulized
lidocaine, but this is not practical for long-term management, and novel
therapies are needed.
There is a need to develop new, more effective
therapies for cough, particularly drugs that act peripherally. There are close
analogies between chronic cough and sensory hyperesthesia, so it is likely that
new therapies are likely to arise from pain research.
DRUGS FOR DYSPNEA
Bronchodilators should reduce breathlessness, and
chronic oxygen may have some effect, but in a few patients, breathlessness may
be extreme. Drugs that have been shown to reduce breathlessness may also
depress ventilation in parallel and may be dangerous in those with severe
asthma and COPD. Some patients show a beneficial response to dihydrocodeine and
diazepam, but these drugs must be used with caution. Slow-release morphine
tablets may also be helpful in COPD patients with extreme dyspnea. Nebulized
morphine may also reduce breathlessness in COPD and could act in part on opioid
receptors in the lung.
VENTILATORY STIMULANTS
Several classes of drug stimulate ventilation and are
indicated when ventilatory drive is inadequate rather than stimulating
ventilation when the respiratory pump is failing. Nikethamide and ethamivan
were originally introduced as respiratory stimulants, but doses stimulating
ventilation are close to those causing convulsions, so their use has been
abandoned. More selective respiratory stimulants have now been developed and
are indicated if ventilation is impaired as a result of over-dose with
sedatives, postanesthetic respiratory depression, and in idiopathic
hypoventilation. Respiratory stimulants are rarely indicated in patients with
COPD because respiratory drive is already maximal, and further stimulation of
ventilation may be counterproductive because of the increase in energy
expenditure caused by the drugs.
Doxapram
At low doses (0.5 mg/kg intravenously), doxapram
stimulates carotid chemoreceptors, but at higher doses, it stimulates medullary
respiratory centers. Its effect is transient, and it must therefore be
administered by intravenous infusion (0.3-3.0 mg/kg/min). The use of doxapram
to treat ventilatory failure in patients with COPD has largely now been
replaced by noninvasive ventilation. Unwanted effects include nausea, sweating,
anxiety, and hallucinations. At higher doses, increased pulmonary and systemic
pressures may occur.
Doxapram is metabolized in the liver and should be
used with caution if hepatic function is impaired.
Acetazolamide
The carbonic anhydrase inhibitor acetazolamide induces
metabolic acidosis and thereby stimulates ventilation, but it is not widely
used because the metabolic imbalance it produces may be detrimental in the face
of respiratory acidosis. It has a very small beneficial effect in respiratory
failure in COPD patients. The drug has proven useful in the prevention of high-
altitude sickness.
Naloxone
Naloxone is a competitive opioid antagonist that is
only indicated if ventilatory depression is caused by overdose of opioids.
Flumazenil
Flumazenil is a CNS benzodiazepine receptor antagonist
and can reverse respiratory depression caused by overdose of benzodiazepines.
Protryptiline
Protryptiline has been used in the treatment of
patients with sleep apnea syndromes, but its mode of action is unclear. It
appears to stimulate activity of upper airway muscles via some central effect.
Modafinil
Modafinil is a nonamphetamine CNS stimulant occasionally used to treat drowsiness in patients with obstructive sleep apnea syndrome as an adjust to continuous positive airway pressure therapy. ide effects include insomnia, anxiety, and tachycardia.











