Membrane Transport Proteins And Ion Channels
Proteins provide several routes for the
movement of materials across membranes:
(i) large pores, constructed of several protein subunits, that allow the bulk
flow of water, ions and sometimes larger molecules (e.g. aquaporin, Chapter 34; and the connexins,
that combine on the connexons to form gap junctions between cells); (ii)
transporter molecules, some of which use metabolic energy (either direct or
indirect) to move molecules against chemical and/or electrical gradients; and
(iii) ion channels, specialized to allow
the passage of particular ion species across the membrane under defined
conditions.
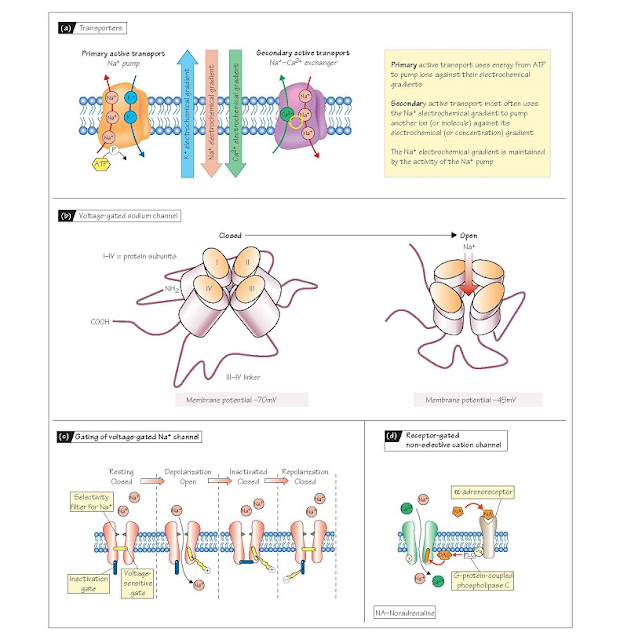
Carrier-mediated transport
Transporter (or carrier) proteins
can move a single type of molecule in one direction across a membrane (a uniporter),
several different molecules in one direction (a symporter) or different
molecules in opposite directions (an antiporter) (Fig. 4a). Transporters
can allow the movement of molecules down chemical concentration gradients (facilitated
diffusion), when the energy required for conformational changes in the
transporter protein is provided by the concentration gradient rather than by
metabolic activity. Important transporters for glucose and amino acids, found
in the kidney and the gut, are in fact driven by the Na+ electrochemical
gradient that exists across the cell membrane (Chapter 2). These symporters
must bind Na+ and the primary transported molecule at the external
surface of the membrane before the conformational change will take place.
Antiporters such as the Na+–Ca2+ exchanger similarly use the Na+ gradient, in
this case to extrude one Ca2+ out of the cell in exchange for three Na+ into
the cell. These processes are known as secondary active transport, as
the Na+ gradient is set up by a process requiring metabolic energy. The uneven
distribution of Na+ ions across the cell membrane is produced by the best known
of all transporters, the Na+–K+ ATPase, also known as the Na+ pump (Fig.
4a). This protein is an antiporter that uses metabolic energy to move Na+ ions
out of the cell and K+ ions in, against their respective concentration
gradients. The ATPase binds extracellular K+ and intracellular Na+ ions,
usually in the ratio of 2:3, and hydrolyses adenosine triphosphate (ATP) to
provide the energy needed to change its conformation, leading to the ejection
of Na+ into the extracellular medium and K+ into the cytosol; this allows the
cell to maintain a high concentration of K+ ions and a low concentration of Na+
ions inside the cell (Chapter 2). The Na+ pump works continuously, although its
activity is stimulated by high intracellular levels of Na+ ions and can be
modulated by second messenger-mediated phosphorylation. The action of the
Na+-K+ ATPase is the most important example of primary active transport.
Ion channels
Ions can diffuse across cell
membranes down their electrochemical gradient through ion channels.
These transmembrane proteins, which are invariably constructed of several
subunits containing several mem- brane-spanning domains (e.g. Fig. 4b), provide
a charged, hydrophilic pore through which ions can move across the lipid
bilayer. They possess a number of
important features that confer upon the cell the ability to control closely the movement of ions across the membrane. Ion
channels are selective for particular ions, i.e. they allow the passage
of only one type of ion or a few related ions. There are numerous specialized
channels for Na+, K+, Cl− and Ca2+ ions, as well as non-specific channels for
monovalent, divalent or even all cations (positively charged ions) or anions
(negatively charged ions). The charge on the transmembrane pore determines
whether the channel is for cations or anions, and selection between different
ion types depends on the size of the ion and its accompanying water of
hydration. Different types of channel for the same ion can however allow
greatly differing amounts of that ion to move through them per second for the
same electrochemical gradient; this is called channel conductance, and
is best understood in the following way. Ions carry charge and so their
movement causes an electrical current. Ohm’s law states that V (voltage) = I (current) × R (resistance). In
terms of ion channels, V = membrane potential and I = ionic current, so one can
calculate the resistance of the
channel. The reciprocal of resistance is conductance, which has units called
Siemens; 1 Siemens (S) = 1/Ohm. Single ion channels generally have conductances
in the 2–300 pS (10–12 S) range.
The second key feature of ion
channels is that their pores are either open or closed; the
transition between these states is called gating. Gating is brought
about by a change in the conformation of the protein subunits that opens or
closes the ion-permeable pore (e.g. Fig. 4b). Many channels are opened or
closed according to the potential difference (voltage) across the cell
membrane (voltage gating; Chapter 5), whereas others are gated by the
presence of a specific signal molecule (ligand or receptor gating).
The function of some channels may additionally be modified by phosphorylation
of channel proteins by enzymes such as protein kinase C or A. The voltage-gated
fast inward Na+ channel that is responsible for the upstroke of the action
potential (Chapter 5) has two gates, one that opens as the cell depolarizes
beyond ∼–55 mV (its threshold)
and another that shuts (inactivates) the channel as the potential becomes positive (Fig. 4c). This latter
gate can only be reset by
repolarizing towards the resting potential (Chapter 5). Some ligand-gated
channels are directly gated by extracellular molecules, such as
neurotransmitters or hormones, whereas others respond indirectly via
intracellular signals, such as diacylglycerol (DAG; Fig. 4d) or cyclic
adenosine monophosphate (cAMP) (Chapter 3). Specialized cells that detect
changes in the internal and external environments (receptor cells) possess ion
channels that are gated by the particular signal that is detected by the
receptor, e.g. pH or light. The characteristics of ion channels, in concert
with the activities of ion pumps, give cells the ability to control precisely
the movement of ions across the cell membrane. This is crucial for many
important physiological processes, including electrical signalling (Chapters 5
and 6), initiation of muscle contraction (Chapters 12 and 13) and the release
of materials such as neurotransmitters, hormones and digestive enzymes.