Primary Amenorrhea
Primary amenorrhea is defined as failure to menstruate by age 16
in patients with normal secondary sexual characteristics or the failure to
menstruate by age 14 in patients with no signs of sexual maturation (Fig.
30.1). Secondary amenorrhea is defined as the absence of three menstrual
cycles or the absence of menstrual bleeding for 6 months.
The distinction between primary and secondary amenorrhea has traditionally been emphasized because of the higher incidence of genetic and anatomic abnormalities among young women with primary amenorrhea. It remains conceptually useful to make this distinction because of several unique disorders that are found only in patients with one or the other. Still, there is much more overlap in the origins and pathophysiology of the two entities than was originally appreciated. For example, Turner syndrome is a common genetic cause of primary amenorrhea, yet some patients with Turner syndrome have sufficient ovarian reserve to undergo secondary sexual development and menarche before complete ovarian failure results in secondary amenorrhea. Other young women with chronic anovulation due to functional disorders will be classified with primary amenorrhea if the onset of the disorder occurs at puberty. In such cases, it may be more useful to assess the degree to which secondary sexual characteristics have developed in girls with absent menses. Failure of breast and pubic hair development is a sign of delayed or absent puberty and represents a specific subset of reproductive abnormalities (Chapter 29).
The distinction between primary and secondary amenorrhea has traditionally been emphasized because of the higher incidence of genetic and anatomic abnormalities among young women with primary amenorrhea. It remains conceptually useful to make this distinction because of several unique disorders that are found only in patients with one or the other. Still, there is much more overlap in the origins and pathophysiology of the two entities than was originally appreciated. For example, Turner syndrome is a common genetic cause of primary amenorrhea, yet some patients with Turner syndrome have sufficient ovarian reserve to undergo secondary sexual development and menarche before complete ovarian failure results in secondary amenorrhea. Other young women with chronic anovulation due to functional disorders will be classified with primary amenorrhea if the onset of the disorder occurs at puberty. In such cases, it may be more useful to assess the degree to which secondary sexual characteristics have developed in girls with absent menses. Failure of breast and pubic hair development is a sign of delayed or absent puberty and represents a specific subset of reproductive abnormalities (Chapter 29).
As Table 30.1 shows, causes of
amenorrhea are extensive and involve all levels of the
hypothalamic–pituitary–gonadal–end-organ axis. To avoid confusion, amenorrhea
can be divided into two broad categories of abnormalities. The first and
largest category is characterized by chronic anovulation. In these patients, a
failure to generate cyclic ovarian estrogen and progesterone leads to absent or
highly irregular sloughing of an inappropriately stimulated endometrium
(Chapters 10 and 14). Chronic anovulation results from four general
pathophysiologic mechanisms: (i) the hypothalamus fails to generate a cyclic
gonadotropin-releasing hormone (GnRH) signal to the pituitary gland; (ii) the
pituitary fails to respond to appropriate signals from the hypothalamus; (iii)
the normal sex steroid feedback mechanisms fail to drive the midcycle
luteinizing hormone (LH) surge; (iv) interference with gonadal steroid feedback
by other endocrine systems. The second, much smaller, category includes
end-organ abnormalities that interfere with the ability of these organs to
respond to normal cyclic ovarian steroid production and produce visible
endometrial bleeding. Diagnosing the underlying cause of amenorrhea involves
sequential determination of the function of each of the potentially affected
compartments (uterus and vagina, ovaries, pituitary and hypothalamus).
Treatment aims to correct the underlying dysfunction so that menses resume. If
it is not possible to establish or restore menstruation, it is very important
to assess the hormonal status of untreated or inadequately treated individuals.
Chronically hypestrogenic women are at increased risk for osteoporosis (Chapter
24) and women with chronic unopposed estrogen stimulation of their endometrium
are at risk for endometrial cancer (Chapter 43). Hormonal therapy to avoid
these consequences must be considered in all
amenorrheic women.
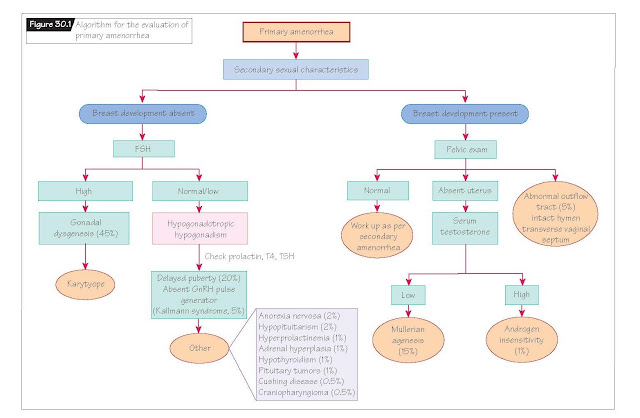
Etiologies of primary amenorrhea
These are best understood if
categorized by: (i) the presence or absence of breast development; (ii) the
presence or absence of the cervix and uterus; and (iii) circulating
follicle-stimulating hormone (FSH) levels. Figure 30.1 presents an algorithm
for evaluating the girl or woman with primary amenorrhea. Unsurprisingly,
abnormalities in each of the four compartments mentioned above can be
associated with primary amenorrhea.
In order of descending frequency,
the most common causes of primary amenorrhea are gonadal dysgenesis,
physiologic delay of puberty, Müllerian agenesis, transverse vaginal septum or
imperforate hymen, Kallmann syndrome, anorexia nervosa and hypopituitarism.
Complete androgen insensitivity, while much rarer than Müllerian agenesis, must
be considered in any young woman who has breasts but no uterus. All girls or
women with primary amenorrhea and an elevated FSH must have a karyotype
performed to determine whether 2X chromosomes are present or if a Y chromosome
(or even a piece of a Y chromosome) is present. The presence of any Y
chromosome genes and an intraabdominal gonad, regardless of its phenotype,
confers a risk for germ-cell tumor development. These gonads must be surgically
removed, typically at the time of diagnosis.
Gonadal dysgenesis with a
pure 45X karyotype can usually be diagnosed because of the other physical
features of Turner syndrome (Chapters 27 and 29). Other abnormalities of the
sex chromosomes can also cause amenorrhea, including 45X/46XX, other mosaics,
and 46XY with a missing SRY locus (Chapter 5). Müllerian agenesis, also
known as the Mayer–Rokitansky–Kuster–Hauser syndrome, is characterized by a
complete absence of the female internal genitalia, including the vagina, uterus
and fallopian tubes, in a chromosomally normal female. Its biologic cause is
unknown. Transverse vaginal septa are thought to result from failure of
the vaginal plate to resorb at the site where the Müllerian ducts fuse with it
to form the cervix (Chapters 6 and 27). Kallmann syndrome is a
developmental abnormality of the central nervous system (CNS) in which those
neurosecretory cells destined to become the GnRH pulse generator fail to
migrate from their origins in the olfactory placode to the median basal hypothalamus
(Chapter 29). In addition to reproductive abnormalities, individuals with
Kallmann syndrome also cannot smell because of the inadequate development or
complete absence of the olfactory neurons that develop from the same anlagen. Anorexia
nervosa or extreme exercise and their consequent hypothalamic suppression
can cause delayed or absent puberty if the disorder begins in childhood,
primary amenorrhea if it begins during puberty, or secondary amenorrhea if it
begins later in adolescence. Hypopituitarism most commonly results from
CNS tumors and can present as either absent or delayed puberty or amenorrhea
depending on timing of onset and the rate of tumor growth. Complete
androgen insensitivity (AI),
previously called testicular
feminization, is a rare X-linked disorder caused by mutations in the
androgen receptor that make it unresponsive to androgen. Although they can make
testosterone and other androgens, patients with complete AI cannot exhibit
androgen activity at central or peripheral target tissues. Genitalia fail to
masculinize during embryogenesis and androgens cannot exert negative feedback
on FSH production by the pituitary gland. Individuals with complete AI are
phenotypic girls and will develop breasts at puberty because the androgens
secreted by their overstimulated testes can be converted peripherally to
estrogens. They do not have a uterus. Therefore, they will not menstruate and
will present with primary amenorrhea in the presence of adequate breast development.