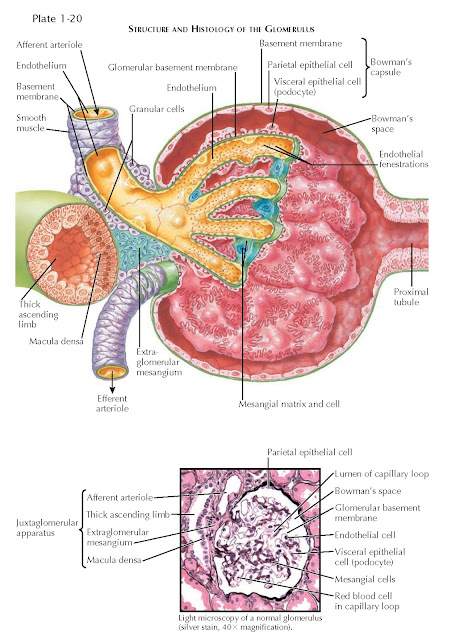
Glomerulus
The glomerulus (or renal corpuscle) consists of the glomerular
capillaries and the epithelium-lined sac that surrounds and invests them, known
as Bowman’s capsule.
The glomerular capillaries originate from the afferent
arteriole and drain into an efferent arteriole. They are arranged in a tuft
about 200 µm in diameter, which is anchored to a central stalk of mesangial
cells and matrix. The walls of the glomerular capillaries contain three layers.
The innermost layer consists of endothelial cells. The second layer consists of
glomerular basement membrane (GBM). The outermost layer consists of podocytes,
also known as visceral epithelial cells.
Bowman’s capsule, the first part of the nephron, consists
of the two layers of epithelial cells that invest the glomerular capillaries.
The podocytes (visceral epithelial cells) in the capillary wall constitute the
inner layer of Bowman’s capsule. The parietal epithelial cells, which are
continuous with the podocytes at the base of the capillary tuft, constitute its
outer layer. The area between the podocyte and parietal epithelial cell layers
is known as Bowman’s space.
The Capillary Wall
As blood passes through the glomerular capillaries,
plasma and small, non-protein bound solutes are freely filtered across the three layers of
the capillary wall into Bowman’s space, which leads to the proximal tubule.
These three capillary wall layers, however, act as a critical barrier to the
filtration of cells and larger plasma molecules, such as proteins, based on
their size and charge.
The endothelial cells, which line the inner surface of
the capillaries, are inconspicuous and possess a thin, attenuated cytoplasm.
Their nuclei are generally located near the mesangial stalk, so as not to interfere
with filtration. These cells contain fenestrations that are approximately 70 to
100 nm in diameter, which may serve as an initial size-based filtration barrier.
The cell surfaces are also coated with a negatively charged glycocalyx that
projects into the fenestrations and provides a charge-based filtration barrier.
The GBM lines the outer surface of the endothelial cells
and is continuous with the basement membrane of Bowman’s capsule. It is
synthesized by both endothelial cells and podocytes, and it consists of three layers: a
thin lamina rara interna, a thick central lamina densa, and a thin lamina rara
externa. Together, these layers measure approximately 300 to 350 nm across,
being somewhat thicker in males than in females. The GBM consists primarily of
type IV collagen and other proteins, such as laminin and nidogen (also known as
entactin). The tight arrangement of these proteins contributes to the
size-based filtration barrier. In addition, the GBM contains negatively charged
proteoglycans that contribute to the charge-based filtration barrier. The potential
space between the endothelial cells and GBM is known as the subendothelial
space, while the potential space between the GBM and the podocytes is known as
the subepithelial space.
The podocytes are large cells with prominent nuclei and
other intracellular organelles. Their cytoplasm is elaborately drawn out into
long processes that give rise to fingerlike projections known as foot processes
(pedicels). These foot processes attach to the outer surface of the GBM and
interdigitate with those from adjacent podocytes. They also lie between the
podocyte cell bodies and the GBM, forming a subpodocyte space. The space
between adjacent foot processes is generally about 25 to 60 nm. A structure
known as the slit diaphragm spans this distance. It consists of an 11 nm-wide
central filament attached to adjacent podocyte cell membranes by cross-bridging
proteins arranged in a zipper-like configuration. The pores formed between the
central filament, cell membranes, and cross-bridges have been measured as
approximately 4 x 14 nm. These small pores in the slit diaphragm make a
critical contribution to the size-based filtration barrier. In addition, the
podocytes are lined by a negatively-charged glycocalyx, which likely contributes
to the charge-based barrier.
The relative contributions of the three layers of the
capillary wall to the filtration barrier remain controversial. The slit
diaphragm is likely the main obstacle to protein diffusion. Indeed, glomerular
diseases that cause loss of protein into the urine (proteinuria) generally
cause a process known as foot process effacement, in which foot processes
retract and shorten, disrupting slit diaphragms and opening a wide space for
the passage of proteins. Nonetheless, disruption of the endothelial layer or
GBM has also been shown to cause proteinuria, suggesting that these layers also
make important contributions.
Additional Cell Types
The mesangial cells provide structural support to the
glomerular capillaries. These cells are irregularly shaped and send long
cytoplasmic processes between endothelial cells. They are similar to modified
smooth muscle cells and stain positive for smooth muscle actin and myosin.
These cells can contract in response to various signals, narrowing the
capillary loops and reducing glomerular flow. Signals that modulate mesangial
tone include angiotensin II (see Plate 3-18), antidiuretic hormone (see Plate
3-17), norepinephrine, and thromboxane. In addition, mesangial cells are
capable of phagocytosing local macromolecules and immune complexes, as well as
generating inflammatory mediators in
response. The mesangial cells are embedded in the mesangial matrix, which
contains collagen, various proteoglycans, and other molecules. In histologic
sections of normal glomeruli, one or two mesangial cells are typically seen per
matrix area, with a greater number seen in certain pathologic states.
The parietal epithelial cells are flat squamous cells
with sparse organelles. They are continuous with the visceral epithelial cells
near the base of the glomerular capillary tuft and with the cells of the
proximal tubule at the opposite side of the glomerulus. In histologic sections
of normal glomeruli, one or two layers of parietal epithelial cells may be
seen. In severe, rapidly progressive glomerular disease, additional layers of
parietal cells may be seen.
The Juxtaglomerular Apparatus
The juxtaglomerular apparatus is a specialized structure
that consists of components from both the glomerulus and the distal tubule of
its associated nephron.
The glomerular components include the terminal afferent
arteriole, initial efferent arteriole, and extraglo-merular mesangium (also
known as the lacis or as the cells of Goormaghtigh). The nephron supplied by
this glomerulus loops around so that its thick ascending limb contacts the
extraglomerular mesangium. The region of the thick ascending limb that makes
direct contact with the extraglomerular mesangium contains specialized cells
and is known as the macula densa.
Because of this arrangement, the distal tubule is able
to provide feedback to the glomerulus to modulate the filtration rate. In the
setting of inadequate tubular flow, for example, the macula densa triggers
dilation of the afferent arteriole, which increases the filtration rate, and stimulates
renin secretion from specialized cells, known as granular cells,
in the walls of the afferent and efferent arterioles. (For details, see Plate
3-18.)
The extraglomerular mesangial cells are continuous with
and resemble normal mesangial cells. They are linked to the granular cells via
gap junctions, and they share a basement membrane and interstitium with the
adjacent macula densa cells. Thus the extraglomerular mesangium appears to serve
as the signaling intermediary between the tubular and vascular components of
the juxtaglomerular apparatus.
The granular cells are similar to ordinary smooth muscle
cells but have sparser smooth muscle myosin and contain numerous renin-filled
vesicles. Because they produce large quantities of hormones, these cells also
feature a prominent endoplasmic reticulum and Golgi apparatus.
Finally, the macula densa cells appear distinct from the
neighboring tubular cells; a detailed description is available on Plate 1-25.