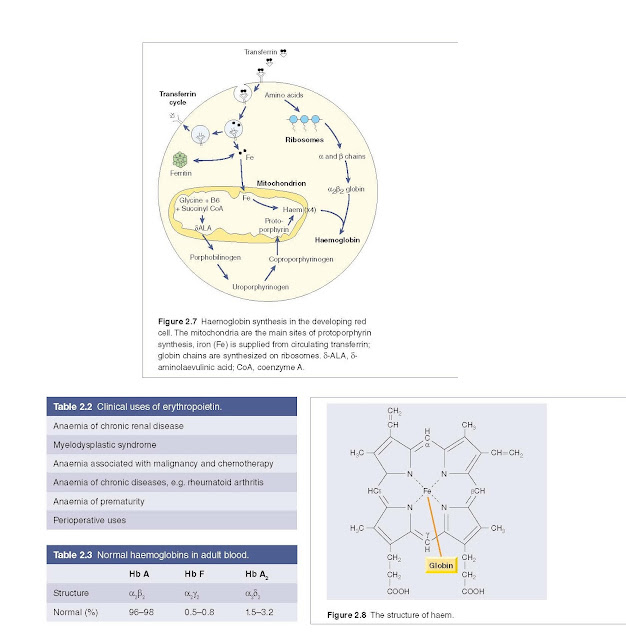
Haemoglobin
Haemoglobin Synthesis
The main function of red cells is to carry O2 to the
tissues and to return carbon dioxide (CO2) from the tissues to the lungs. In order
to achieve this gaseous exchange they contain the specialized protein
haemoglobin. Each molecule of normal adult haemoglobin A (Hb A) (the dominant
haemoglobin in blood after the age of 3–6 months) consists of four polypeptide
chains, α2β2, each with its own haem group. Normal adult blood also contains
small quantities of two other haemoglobins: Hb F and Hb A2. These also contain
α chains, but with γ and δ chains, respectively, instead of β (Table 2.3). The
synthesis of the various globin chains in the fetus and adult is
discussed in more detail in Chapter 7.
Haem synthesis occurs largely in the mitochondria by a series of
biochemical reactions commencing with the condensation of glycine and succinyl coenzyme A
under the action of the key rate‐limiting enzyme δ‐aminolaevulinic acid (ALA)
synthase (Fig. 2.7). Pyridoxal phosphate (vitamin B6) is a coenzyme for this
reaction. Ultimately, protoporphyrin combines with iron in the ferrous (Fe2+)
state to form haem (Fig. 2.8).
The red cells in
systemic arterial blood carry O2 from the lungs to the
tissues and return in venous blood with CO2 to the lungs. As the
haemoglobin molecule loads and unloads O2 the individual globin
chains move on each other (Fig. 2.9). The α1β1 and α2β2 contacts stabilize the
molecule. When O2 is unloaded the β chains are pulled apart, per-
mitting entry of the metabolite 2,3‐diphosphoglycerate (2,3‐DPG) resulting in a
lower affinity of the molecule for O2. This movement is responsible
for the sigmoid form of the haemoglobin O2 dissociation curve (Fig.
2.10). The P50 (i.e. the partial pressure of O2 at which
haemoglobin is half saturated with O2) of normal blood is 26.6 mmHg.
With increased affinity for O2, the curve shifts to the left (i.e.
the P50 falls) while with decreased affinity for O2, the
curve shifts to the right (i.e. the P50 rises).
Normally, in vivo, O2 exchange
operates between 95% saturation (arterial blood) with a mean arterial O2
tension of 95 mmHg and 70% saturation (venous blood) with a mean venous O2
tension of 40 mmHg (Fig. 2.10).
The normal position of the curve depends on the concentration
of 2,3‐DPG, H+ ions and CO2 in the red cell and on the structure of
the haemoglobin molecule. High concentrations of 2,3‐DPG, H+ or CO2,
and the presence of sickle haemoglobin (Hb S), shift the curve to the right
(oxygen is given up more easily) whereas fetal haemoglobin (Hb F) – which is
unable to bind 2,3‐DPG – and certain rare abnormal haemoglobins associated with
polycythaemia shift the curve to the left because they give up O2 less readily
than normal.
Methaemoglobinaemia
This is a clinical state in which circulating
haemoglobin is present with iron in the oxidized (Fe3+) instead of the
usual Fe2+ state. It may arise because of a hereditary deficiency of
methaemoglobin reductase deficiency or inheritance of a structurally abnormal
haemoglobin (Hb M). Hb Ms contain an amino acid substitution affecting the haem
pocket of the globin chain. Toxic methaemoglobinaemia (and/or sulphaemoglobinaemia)
occurs when a drug or other toxic substance oxidizes haemoglobin. In all these
states, the patient is likely to show cyanosis.