Assessment For Kidney Transplantion
Although renal transplantation improves both quality of life and survival,
it involves a significant investment of health resources and the use of an
organ with a limited supply. It is therefore of utmost importance that the
potential transplant recipient is carefully assessed, both to avoid unnecessary
exposure to the risks of a general anaesthetic and to ensure appropriate use of
a precious resource. To this end, every potential transplant recipient is assessed
by taking a careful history, performing a thorough examination and undertaking
a number of investigations.
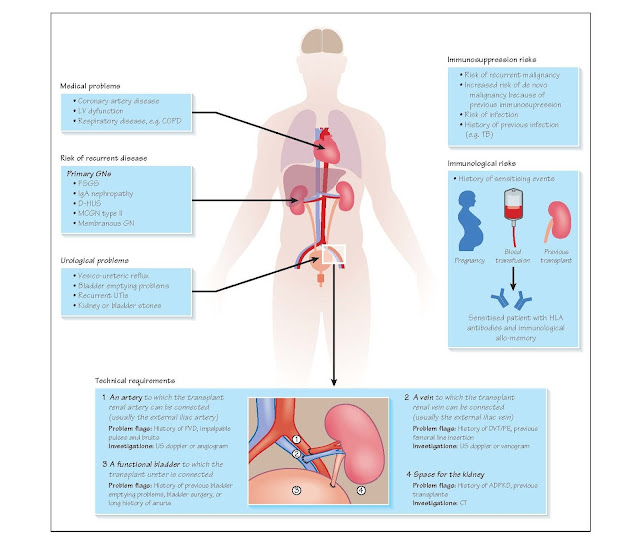
The transplant work-up must answer five questions.
1 Does the patient have any medical problems which put
them at risk of operative morbidity/mortality? Patients with CKD are at increased risk of coronary, cerebral and
peripheral vascular disease, and should be assessed for a past or current
history of cardiac problems (e.g. angina, myocardial infarction, rheumatic
fever), strokes or peripheral vascular disease (claudication/amputation). Risk
factors assessed include family history, smoking history and a history of
diabetes mellitus or hypercholesterolaemia. Smoking is also associated with the
development of chronic obstructive pulmonary disease (COPD). A good screening
question to assess general cardiorespiratory fitness is to ask how far the
patient can walk; a good test is to make them walk.
Dialysis patients are frequently oligo-anuric and often
struggle to restrict their fluid intake. This leads to chronic volume overload
and hypertension, resulting in left ventricular hypertrophy (LVH) or
dysfunction. Patient who require 3–4 litres of fluid to be removed at each
dialysis session frequently develop such cardiac problems.
CKD is also associated with tertiary hyperparathyroidism
and hypercalcaemia, which increases the risk of vascular and valvular
calcification, particularly the aortic valve.
Examination should pay particular attention to
cardiovascular signs: pulse rhythm and volume, signs of volume overload (ele-
vated jugular venous pressure [JVP], peripheral and pulmonary oedema), signs of
LVH (hyperdynamic apex beat) or LV dilatation (displaced apex beat) and signs
of valvular heart disease (particularly the ejection systolic murmur of calcific
aortic stenosis). The chest should be assessed for signs of COPD
(hyperinflation, reduced expansion, wheeze) or for pleural effusions which may
occur in patients on peritoneal dialysis.
Cardiorespiratory investigations include an
electrocardiogram (ECG), a chest radiograph, a cardiac stress test (an exercise
tolerance test or an isotope perfusion study) and an echocardiogram (to assess
LV function). If these are abnormal, then the patient may need further
cardiological assessment, including coronary angiography.
2. Does the patient have any conditions that make them
technically difficult to transplant?
There are four basic technical requirements for
implantation of a kidney.
· An artery (usually the external
iliac artery), to which the trans- plant renal artery will be anastomosed.
Severe vascular disease can make the arterial anastomosis difficult, therefore
all of the patient’s lower limb pulses should be carefully assessed during
examination, including auscultation of the femoral arteries and aortic bifurca-
tion for bruits, as a surrogate for iliac artery disease. Duplex imaging is
indicated if any abnormality is detected or suspected.
· A vein (usually the external iliac vein), to which
the transplant renal vein will be anastomosed. A history of venous thromboem-
bolic disease, particularly clots in the lower limb veins, should be sought; a
transplant should not be placed above a limb where a thrombosis has occurred
previously. Patients on chronic haemo- dialysis may have had numerous lines
inserted into their femoral veins, which can lead to stenosis and thrombosis.
Look for col- laterals, cutaneous signs of venous hypertension and oedema,
which may be associated with venous compromise. Duplex imaging or percutaneous
venography may be required.
· A bladder, to which the transplant
ureter will be anastomosed. A history of urological problems, including
congenital bladder malformations or reflux, is of relevance. If these issues
are not resolved prior to transplantation, then they may recur and damage the
transplanted kidney. Patients who have had ESRF for a number of years often
have negligible urine output and a small, shrunken bladder, which is difficult
to find intra-operatively and will only hold small volumes of urine post
transplant. Some patients need a neobladder fashioned from a segment of their
ileum (a urostomy).
· Space for the kidney. Some
patients with polycystic kidney disease have grossly enlarged native
kidneys that extend into the lower abdomen and may require removal prior to
transplantation. In addition, patients with an elevated body mass index (BMI)
may be technically difficult to transplant, due to lack of space for the graft
and reduced ease of access to the vessels. Therefore, most centres will not
list patients for transplantation unless the BMI is <35 kg/m2.
3. Is the patient at increased risk of the immunological complications of
transplantation?
The immune system remains a significant barrier to
transplantation in patients with pre-formed antibodies to non-self human
leucocyte antigens (HLA). This usually occurs as a result of a sensitising
event, for example blood transfusion, pregnancy (particularly by multiple
partners), or previous renal transplants or other allografts (e.g. skin
grafts). The frequency of such events should be ascertained.
4. Is the patient at increased risk of immunosuppression-associated
complications?
Patients with ESRF secondary to a primary or secondary
glomerulonephritis (e.g. IgA, vasculitis or lupus) have frequently been treated
with immunosuppressants. This includes the use of toxic agents, such as
cyclophosphamide, or biological agents, including alemtuzumab or rituximab.
Heavy immunosuppression should be avoided in such patients post-transplant,
particularly the use of lymphocyte-depleting agents such as anti-thymocyte
globulin (ATG), which may place them at high risk of infectious complications.
Immunosuppression also increases the risk of developing a
de novo cancer (particularly oncovirus-associated malignancies), and
enhances the progression of existing cancers. Thus, most centres would agree
that patients with a history of malignancy must be cancer-free for at least 5
years prior to transplantation.
5. Is the patient at risk of recurrent disease in their transplant?
Some pathologies that cause CKD can recur in the
transplant and reduce its long-term function and survival. A number of
glomerulonephritides can affect the graft (e.g. IgA nephropathy and focal
segmental glomerulosclerosis [FSGS]). In the case of FSGS, the patient may
develop recurrent disease immediately post transplant (usually evidenced by
heavy proteinuria). This is sometimes amenable to treatment with plasma
exchange, therefore it is important to recognise this risk and carefully
monitor the patient post transplant. If a patient has developed rapidly
progressive, recurrent disease in a transplant kidney, then this is a relative
contraindication to re-transplantation.