DEVELOPMENT
OF THE LOWER RESPIRATORY SYSTEM
The development of the
respiratory system in humans is an interesting demonstration of ontogeny
recapitulating phylogeny. The embryology of the system goes through the fish,
amphibian, reptilian, and mammalian evolutionary stages of humans’ ancestry. In
the change from an aqueous to an aerobic environment, many basic structures
were modified but retained as parts of the respiratory system, and others became
nonrespiratory structures. At the same time, entirely new respiratory
structures evolved. The olfactory organ of aqueous forms was incorporated into
the respiratory system of terrestrial forms, and the simple sphincter mechanism
of the swim bladder of fish became the larynx of air breathers, which also took
on the function of phonation. In contrast, the part of the respiratory system
involved in the gas exchange vital to life has essentially not changed
throughout vertebrate evolution. Exchange of oxygen and carbon dioxide between
the external environment and the circulating bloodstream occurs through a wet
epithelium in both gills and lungs.
The respiratory system in
humans differs from the other major body systems in that it is not operational
until birth. Therefore, development of the antenatal respiratory system is
genetically determined independently of the functional demands of the growing
embryo and fetus. The system’s physiologic development is mainly one of preparation
for instant action at birth, a feat unmatched by any other system. When the
fetus passes from the uterine aquatic environment, the partially collapsed,
fluid filled lungs immediately function efficiently to sustain life. The chief
cause of perinatal death of human infants is failure of the respiratory system
to work properly. In the majority of perinatal deaths, all other body systems
are functioning normally.
Primitive Respiratory Tube
During the fourth gestational
week, the first indication of the future respiratory tree is a groove that runs
lengthwise in the floor of the pharynx just caudal to the pharyngeal pouches.
From the outside, this laryngotracheal groove appears as a ridge. The ridge
grows caudally to become a tube, the lung bud, and the cranial or upper part of
the tube becomes the larynx. The caudal part becomes the future trachea, which
soon develops two knoblike enlargements at its distal end, the bronchial buds
(Plate 1-33).
Trachea
As the trachea lengthens,
anterior to and parallel with the esophagus, the bronchial buds are carried progressively
more caudal in the body until they reach their definitive position in the
thorax. During this growth period, mesenchymal cells from the splanchnic
mesoderm surround the tracheal tube of entoderm and give rise to the connective
tissue, smooth muscle, and cartilage of the tracheal wall. By and during the
eighth gestational week, the rudiments of the 16 to 20 C-shaped tracheal
cartilages appear (see Plate 1-36). These mesenchymal rudiments transform into
cartilage in a cranial to caudal
direction up to the tenth week. Only the epithelial lining and glands of the
trachea are derived from entoderm. The lining starts to become ciliated at 10
weeks, with the cilia beating toward the larynx. By 12 weeks, the mucosal
glands begin to appear in a cranial to caudal direction. All major microscopic
features are recognizable by the end of the fifth month. However, the infantile
trachea differs grossly from the adult form because it is short and narrow
compared with a relatively very large larynx. This size difference continues
for several months after birth.
Bronchi
The bronchial buds of the
trachea become the two main bronchi. As soon as the right bronchus appears, it
is a little larger than the left one and tends to be more vertically oriented
(see Plates 1-33 and 1-36). These differences become more pronounced up to and
after the time the bronchi mature, accounting for the fact that foreign bodies
enter the right main bronchus much more often than the left.
During the fifth week, each
main bronchus gives rise to two bronchial buds. These buds develop secondary
branches to the future lobes: the upper, middle, and lower lobes on the right
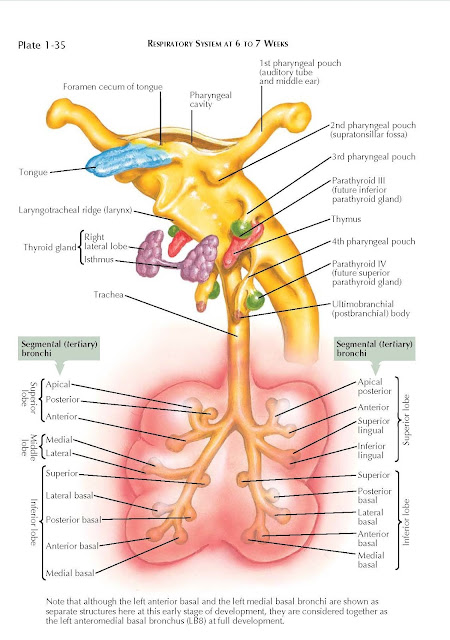
side and the upper and lower lobes on the left (Plate 1-34). By the seventh week,
tertiary branches appear (see Plate 1-35), 10 in the right lung
and nine in the left. These tertiary branches will supply the clinically
important broncho- pulmonary segments, which become separated from each other
by tenuous connective tissue septa (see Plate 1-36). The tenuous connective
tissue surrounding each segment delineates a separate respiratory unit of the
lung, but some collateral ventilation does occur between segments. A branch of
the pulmonary artery accompanies each segmental bronchus to serve as the
independent blood supply to a bronchopulmonary segment. Again, some collateral
circulation occurs across segments. The pulmonary veins do not accompany the
segmental bronchi and arteries but run chiefly through the substance of the lung
between the segments, as do the lymphatic vessels.
Branching of the segmental
bronchi continues until, by the sixth month, about 17 orders of branching have
been formed. Additional branching continues postnatally and until puberty, when
about 24 orders of branches have been established. After the full complement of
branches has appeared, no new ones will form to replace any lost through trauma
or disease. The mature lung makes up for any branches lost by enlarging the
remaining functional segments, which then do more work (compensatory
hyperinflation).
Cartilage, Smooth Muscle,
And Connective Tissue
Cartilage is present in the
main bronchi by the tenth week and in the segmental bronchi by the twelfth
week.
Cilia appear in the lining of the main bronchi at 12 weeks and in the
segmental bronchi at 13 weeks. At birth, the ciliated epithelium extends to the
terminal bronchioles.
Mucous glands appear in the
bronchi at 13 weeks and actively produce mucus by 14 weeks. At 28 weeks,
seven-eighths of the potential adult number of mucous glands is present in the
respiratory tubes.
By the third gestational
month, smooth muscle cells differentiate to form the posterior wall of the
trachea and extrapulmonary main bronchi, which permanently lack cartilage.
Smooth muscle cells form bundles arranged obliquely and circularly around the
bronchi- oles, including the terminal bronchioles, whose entire walls have no
cartilage. The smooth muscle that extends to the alveolar ducts acts as a
sphincter. In an allergic reaction, such as bronchial asthma, smooth muscle
spasm greatly increases airway resistance. High surface tension in the terminal
airways containing a large accumulation of mucus then further reduces the
smaller than normal bronchiolar diameter during expiration. Because inspiration
is affected by contraction of powerful muscles and is associated with widening
and lengthening of the bronchial tree muscles, individuals with asthma can
usually inspire adequately. But these individuals have great difficulty exhaling
because expiration normally results from passive recoil of the stretched
thoracic wall and lungs. To overcome the increased airway resistance of an
asthmatic attack, muscles of the anterior abdominal wall must be contracted and
stabilized, thus allowing the diaphragm to push with greater force and drive
air out of the lungs with maximum effort.
Autonomic innervation of the
lungs is not extensive; all effects of both sympathetic and parasympathetic
innervation are mild. Parasympathetic stimulation can cause moderate
contraction of smooth muscle of the respiratory tubes and perhaps some
dilatation of the blood vessels. In contrast, sympathetic stimulation may
mildly dilate the tubes and mildly constrict the vessels. Therefore,
sympathomimetic drugs may be helpful in inhibiting the spasmodic contraction of
the respiratory tube smooth muscle during an asthmatic attack.
Pleural Cavities
The pericardial, pleural, and
peritoneal cavities develop as subdivisions of two primitive coclomic cavities
that extend along the length of the embryo. Normally, each is only a potential
space with serous lining that produces a slimy secretion. This reduces friction
as the ordinarily apposed surfaces rub against each other. After trauma or
other forms of pathology, the cavities may become actual spaces containing
proteinaceous exudate, air, or blood.
During the second week of
life, the two coelomic cavities in the region of the developing heart fuse into
a single pericardial coelom. While the pericardial cavity is becoming established,
it is in open communication caudally on each side with the still paired
primitive coeloms in the embryo’s future abdominal region. Partitioning of the
pericardial coelom from these primitive coeloms starts by
the establishment of a shelf of mesenchyme, the transverse septum, into which
the liver becomes incorporated as it is developing (see Plate 1-34). This
transverse septum grows in from the anterior body wall toward the dorsal or
posterior body wall but never reaches it and finally becomes part of the
diaphragm. Therefore, the two channels of communication between the pericardial
coelom and the two primitive coelomic cavities persist to become the pleural
canals.
Pleural Canals
In the fish stage of vertebrate
evolution, the transverse septum completely separates the pericardial and
peritoneal cavities. Whereas in lungfish the air bladder projects directly into
a common pleuroperitoneal space, in amphibians and reptiles the lungs are found
in a similar space caudal to the pericardial cavity. In humans, the amphibian
and reptilian evolutionary stage of lung development occurs when the growing
lungs project into the pleural canals. Each pleural cavity then becomes
isolated by the growth of the pleuropericardial and pleuroperitoneal folds.
These in turn become associated with the transverse septum (see below).
Pleuropericardial and
Pleuroperitoneal Folds
The vertically oriented
pleuropericardial folds arise on each side from the body walls where the common
cardinal veins swing around to enter the sinus venosus, which subsequently
becomes the right atrium. These body-wall folds bulge into the pleural canals
between the lungs and the heart (see Plates 1-34 and 1-38). When the free
borders of the pleuropericardial folds fuse with midline mesenchymal tissue at
the base of the heart, they completely separate what is now the pericardial
cavity from the pleuroperitoneal coelom (see Plate 1-38). At this time, the
latter space contains the lungs as well as the abdominal and pelvic viscera.
The pleuroperitoneal folds are
actually two horizontally oriented ridges of the dorsolateral body wall where
the common cardinal veins are located (see Plate 1-34). Each fold grows
anteriorly and medially to fuse with the transverse septum and mesenchymal
tissue surrounding the aorta, esophagus, and inferior vena cava.
The two pleural canals are
then walled off from the newly formed peritoneal cavity, and the formation of
the pleural cavities and diaphragm is completed (see Plates 1-37 and 1-39).
Diaphragm
A diaphragm is lacking in fish,
amphibians, reptiles, and birds. In mammals, it is the principal respiratory muscle.
Although there are numerous accessory respiratory muscles, they cannot support
life to a normal degree without a functioning diaphragm. Reptiles have a dual
muscular respiratory mechanism: the action of the trunk muscles creates
negative pressure, and the floor of the mouth pushes air into the lungs under
positive pressure. The reptilian action of the muscles of the floor of the mouth
is also the chief respiratory muscular mechanism in amphibians (“frog
breathing”).
In birds, which like mammals
evolved from reptiles, respiration is accomplished chiefly by the intercostal
trunk muscles that move the ribs, to which the lungs are attached.
In the evolutionary transition
from gill breathing to lung breathing, original muscles from the mandibular
arch gave rise to the musculature of the floor of the mouth, especially the
mylohyoid muscle. In amphibians and reptiles, air brought in through the nares
is forced into the lungs by the musculatory action of the floor of the mouth. In
mammals, a new respiratory muscle the diaphragm evolved from structures lacking
muscle in certain reptiles, specifically, the transverse septum and two unfused
coelomic folds that are the pleuroperitoneal folds in mammalian development.
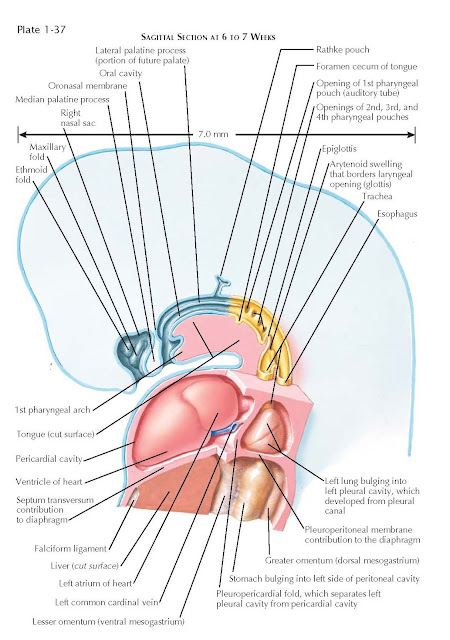 |
| SAGITTAL SECTION AT 6 TO 7 WEEKS |
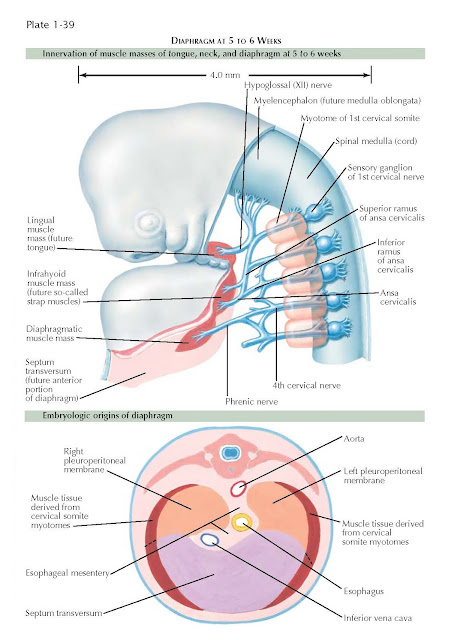
Diaphragmatic musculature in
mammals develops from a common mass of mesoderm at the posterior region of the
branchial arches from which the tongue and infrahyoid muscles are also derived
(see Plate 1-39). The transverse septum, the largest single contribution to the
diaphragm, develops in the neck or cervical region of the embryo (see Plates
1-34 and 1-39). The diaphragmatic striated musculature migrates to the
transverse septum along with branches of the third, fourth, and fifth cervical
spinal nerves, which become its exclusive motor nerve through the phrenic
nerve. By differential growth, especially an increase in size of the thoracic
region, there is a so-called migration and descent of the diaphragm to a much
more caudal position. At the end of the eighth gestational week, the diaphragm
is attached to the dorsal body wall at the level of the first lumbar segment.
The phrenic nerves, which are located in the body wall where the
pleuropericardial folds develop, lengthen as the diaphragm descends. They are,
therefore, relocated to a position between the pericardium and the pleurae as
the pleural cavities increase in size (see Plate 1-38).
After the transverse septum,
the two pleuroperitoneal folds and the numerous other minor folds unite to
complete the diaphragm at or during the seventh gestational week, the
diaphragmatic musculature becomes peripherally positioned (see Plate 1-39), and
its domelike central area remains tendinous. As soon as the diaphragm is
completely developed, it begins to contract at irregular intervals. Near term,
these contractions, which are essentially hiccups, become more vigorous and
more frequent. They exercise the muscles for the time when air breathing begins
at birth.
During inhalation, the
diaphragm flattens as it contracts. This action reduces the intrathoracic
pressure by enlarging the thoracic cavity and with it the intrapulmonary space.
The vocal folds are separated, and thus air rushes into the lungs at
atmospheric pressure. Normal inspiration is caused chiefly by the contraction of
the diaphragm. Other powerful striated muscles that assist the diaphragm are in
the neck and chest region and are attached to the skull, clavicle, ribs,
vertebral column, and upper limbs. Therefore, whereas inspiration is effected
by the contraction of powerful muscles, expiration is largely a passive action
caused by recoil of the stretched tissues of the thoracic wall and lungs.
The diaphragm is subject to
developmental defects that permit herniation of abdominal viscera into the thorax.
The most common diaphragmatic congenital hernia is related to defective
development of the left pleuroperitoneal fold (see Plate 1-39).
Pleura And Mediastinum
The lungs develop much later
than the heart, as was the case throughout their evolutionary history. The
small lungs, posterior to a relatively very large heart, grow in an anterior
direction on each side of it (Plate 1-38). The pleural cavities open in advance
of the growing lungs so they are already prepared to receive them. By the
eighth gestational week, the lungs are larger than the heart and nearly
surround it. The pleural cavities now occupy the two sides of the thoracic
cavity. All other thoracic viscera, including the heart, great vessels,
esophagus, and associated connective tissue, are now between the two pleural
cavities, from the vertebral column to the sternum. This broad medial septum of
viscera and connective tissue is known as the mediastinum.
As the lungs protrude into the
pleural canals (see Plate 1-34), they are invested by the lining mesothelium of
these spaces, which becomes the visceral pleura (Plate 1-38). Before the
pleuropericardial folds wall off the pleural canals from the pericardial
coelom, the mesothelium lining the walls of these thoracic subdivisions is
continuous (see Plates 1-34 and 1-38). As soon as the pleural canals become the
pleural cavities, the lining of the walls of the canals becomes the parietal
pleura. The region where the visceral pleura reflects off the lungs and becomes
continuous with the parietal pleura shifts medially and becomes smaller to
envelop the structures that constitute the root of the lung.
Throughout human development,
the right lung is larger than the left, as is the case with the right and left
pleural cavities. This size differential is related to the shift of the heart
to the left side of the thorax. In adult mammals and reptiles, the right lung
is also larger than the left lung. In adult humans, the space occupied by the
heart produces the cardiac notch of the left lung.
Terminal Respiratory Tubes
The amphibian stage of
development of portions of the respiratory tubes occurs at 4 to 5 weeks when
the bronchial buds are present (see Plate 1-33). Amphibian lungs are
essentially two air sacs, each with a large single lumen. In reptilians,
segmental bronchi are present at 7 to 8 weeks (see Plate 1-36). The reptilian
lung has branching respiratory tubes ending in terminal sacs that are similar
to mammalian primitive alveoli. They add greatly to the surface area where gas
exchange occurs; in contrast, the amphibian lung has only rudimentary alveoli.
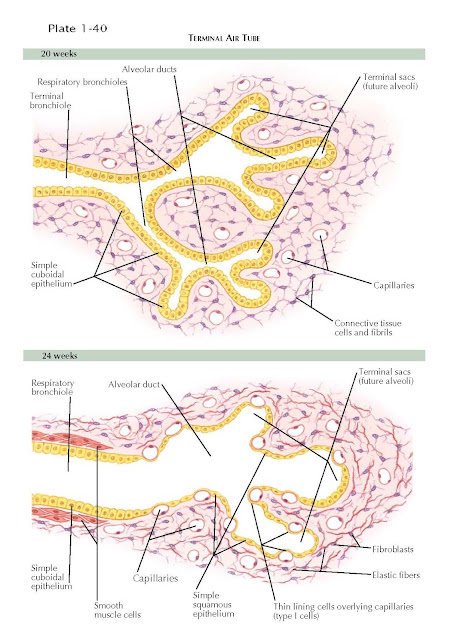
Alveolar development does not
begin in human fetuses until airway development is complete at 16 weeks. Between
the fourth and sixth months of gestation, the last airway is transformed to a
terminal or respiratory bronchiole. Generally, each respiratory bronchiole
divides into three to six alveolar ducts (see Plate 1-40). Each alveolar duct
first ends in a bulging terminal sac lined by cuboidal or columnar epithelium
that ultimately evolves into definitive alveoli. Capillaries multiply so that
the region of terminal airspaces becomes highly vascularized.
During the sixth gestational
month, the epithelium of the terminal sacs thins where it is in contact with a
capillary (see Plate 1-40).The epithelial cells become so thin when the alveoli
fill with air that, before the advent of electron microscopy, there seemed to be
breaks in the lining where only capillary endothelium separated the blood from
the alveolar air (see Plate 1-41). The capillaries, covered by the thin
epithelial cells, line the alveolar spaces (see Plate 1-41). These very thin
cells, constituting the major part of the alveolar surface, are known as type I
pneumocytes. Other cells, scattered along the lining of the alveoli, are
cuboidal, have microvilli on their luminal surfaces, and contain osmiophilic
inclusions of surfactant or its precursors. These cells are known as type II
pneumocytes, and they also appear during the sixth gestational month.
The original mesenchyme that
gives rise to the pulmonary capillaries and lymphatics is also the source of
the fibrocytes that produce an abundance of elastic fibers in the lungs (see
Plate 1-40). After the lungs become inflated with air, the elastic fibers are
constantly stretched and, by attempting to contract, contribute to the normal
recoil or collapsing tendency of the lungs. On the other hand, the natural
tendency of the chest wall is to expand. The resulting negative pressure in the
pleural cavities helps to keep the lungs expanded. The visceral pleurae
continually absorb fluid so that only a small amount of it remains in the
potential intrapleural space at all times. Because the elastic fibers of the
lungs are stretched even more during inspiration, they are the chief structures
responsible for returning the enlarged alveoli and bronchioles to their more
contracted resting dimensions during normal passive expiration.
Alveolar-Capillary
(Respiratory) Membrane
By the 28th week, the lung has
lost its glandular appearance. The respiratory airways end in a cluster of
large thin-walled sacs separated from one another by a matrix of loose
connective tissue. At this stage, respiration can be supported because gas
exchange can occur at the terminal sacs, and surfactant is present to maintain
alveolar stability. The primitive alveoli do not become definitive as true
alveoli until after birth, at which time they are only shallow bulges of the
walls of the terminal sacs and respiratory bronchioles. Even so, the thickness of
the blood-air barrier, which is also known as the respiratory or alveolar-capillary
membrane, is about 0.4 μm. This is within the range found in
adults that is, 2.5 μm to smaller than 0.1 μm (1 μm is
0.001 mm). The lungs of a newborn infant contain 24 million primitive alveoli
(see Plate 1-41).
During the first 3 years of
life, the increase in lung size is caused by alveolar multiplication rather
than by greater alveolar size. From the third to the eighth year, the alveoli
increase in size as well as in number until there are 300 million in the two
lungs. After the eighth year, alveoli become larger only until the chest wall
stops growing. At age 8 years, the diameter of the mature alveolus is 100 to
300 μm. Physical diffusion of oxygen from the alveolus into the red blood cell
and of carbon dioxide in the opposite direction occurs through the respiratory
membrane, which consists of an alveolar type I pneumocyte and a capillary
endothelial cell and their respective basement
membranes. Consequently, oxygen and carbon dioxide do not have to pass across a
great distance between the erythrocyte and the alveolus, and gas diffusion can
be accomplished very rapidly. The total surface area of the respiratory
membrane of both lungs is about 70 m2, which is vast when compared
with the 1.7 m2 of total body surface of an adult. The average
diameter of a pulmonary capillary is only about 7 μm (see Plate 1-41). The
extensive alveolar and associated capillary endothelial surface is also
responsible for a large water vapor loss during respiration; adult lungs
eliminate about 800 mL of water a day in expired air.
Surfactant
No matter how complete the
development of the respiratory system at birth, one factor that determines
whether it will support life is the presence of a substance known as pulmonary
surfactant. Therefore, because of its functional implications, the most
important morphologic event is the appearance at about the twentythird week of
lamellar inclusion bodies in the type II pneumocytes of the lining of the
terminal sacs. These bodies are precursors of surfactant, a lipoprotein mixture
rich in phospholipids, especially dipalmitoyl lecithin. Surfactant has a
“detergent” property of lowering surface tension in the fluid layer that lines
the primitive alveoli after air enters the lungs, and it acts as an
antiatelectasis factor to maintain patency of terminal airspaces (see Plate
1-41).
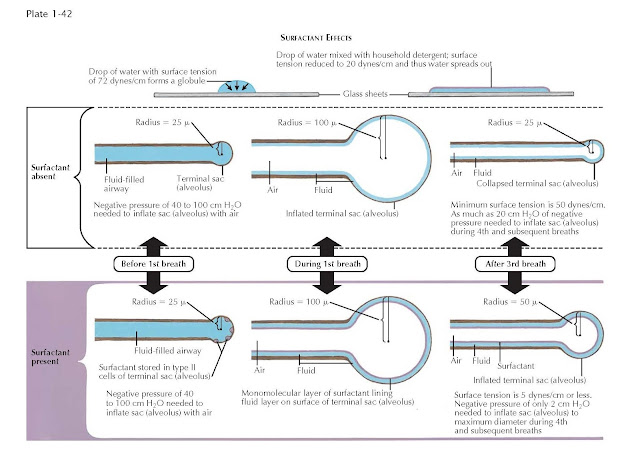
Surface tension of fluid is
measured in dynes per centimeter. A drop of water on a sheet of glass tends to
round up into a compact mass because of its surface tension of about 72
dynes/cm at the air water interface. If household detergent is added to the
drop of water, its surface tension is reduces to about 20 dynes/cm, and it
spreads into a very thin film on the glass (see Plate 1-42). In a similar
manner, surfactant reduces surface tension of the fluid layer lining the
alveolus to about 5 dynes/cm. Its ability to form a monomolecular layer at the
interface between air and the alveolar lining fluid (see Plate 1-41) allows some
air to be retained within the alveolus at all times.
Although surfactant is present
in the lungs as early as the twenty-third gestational week, the lungs at this
stage are unable to retain air after inflation, and they collapse completely
before 28 to 32 weeks. The quantity of surfactant within the lungs increases
markedly toward term; this is one of the most important reasons why older
fetuses have a better chance of survival as air breathers. Surfactant must be
produced continually because it has a half-life of 14 to 24 hours. A deficiency
of surfactant is associated with the infant respiratory distress syndrome
(RDS), also known as hyaline membrane disease (see Plates 4-144 and 4-145).
This is caused by the relative instability of the immature lung because of
failure to produce surfactant in amounts sufficient for neonatal respiration.
Death from the disease occurs within a few hours to a few days after birth. The
alveoli of the dead infants are filled with a proteinaceous fluid that resembles
a glassy or hyaline membrane.
The high incidence of RDS in
premature infants is caused by their low initial concentrations of surfactant.
Prematurity,
cesarean section, and perinatal asphyxia are recognized predisposing factors.
Surface tension of lung extracts of newborn infants with birth weights of 1200
g or more is only about 5 dynes/cm. In extracts from infants with birth weights
less than 1200 g who have hyaline membrane disease, it may be four times that
value.
Before birth, the respiratory
tubes are filled with fluid, some of it amniotic fluid brought in by “practice”
inspiratory movements. However, most of the fluid is produced by the lining of
the respiratory tubes (as much as 120 mL/h near term). This pulmonary fluid
passes through the oral and nasal cavities to mix with the amniotic fluid.
Amniotic fluid contains phospholipids, and amniocentesis before the thirty-fifth
week usually shows that the ratio of lecithin to sphingomyelin is less than or
equal to 1 because the latter remains constant as gestation advances. Such a
ratio indicates that the fetus is immature in regard to surfactant production.
A ratio of more than 2 : 1 indicates that the fetal lungs are sufficiently
mature to prevent the development of RDS.
The role of thyroxine and adrenal
corticosteroids in stimulating lung maturation and surfactant production has
not yet been settled and is still under investigation. Surfactant is present in
the lungs of all vertebrate air breathers. The amount of surfactant correlates
well with alveolar surface area and with the amount of certain saturated
phospholipids in the lung tissue in a stepwise fashion up the phylogenetic
scale from amphibians through reptiles to mammals.
First Breath
Before the first breath, the
lungs are filled with fluid. Therefore, the lungs of a stillborn infant who has
not taken a breath of air differ from those of an infant who has. The lungs of
a stillborn infant are firm; do not crepitate when handled; and because they
contain no air, sink in water. Some of the fluid normally within the lungs at
birth is extruded from the mouth; most of it is removed through the lymphatic
vessels in the region of the primitive alveoli. The pleural lymphatic vessels
are relatively larger and more numerous in fetuses and newborn infants than in
adults, and lymph flow is high during the first few hours after birth. The flow is
less 2 days later but is still higher than in adults.
A certain amount of fluid must
of necessity always remain in the alveoli, but in the partially atelectatic (collapsed)
primitive alveoli, the surface tension of the viscid fluid tends to hold the
walls of the alveoli together. Therefore, the first breath of some 30 to 40 mL
in volume requires a tremendous physical effort, and a negative intrathoracic
pressure as much as 40 to 100 cm of water is needed for expansion. This is
about 14 times the pressure required to produce breaths of a similar volume
subsequently (see Plate 1-42).
Contraction of the diaphragm
is mainly responsible for the first breath that is often associated with the
first good cry, but the accessory muscles of respiration offer little assistance
at this time. Expansion of the chest wall is slight in the days just after
birth. In fact, the thoracic skeleton contains so much flexible cartilage that
the chest wall tends to collapse ith each inspiration, especially in premature
infants.
When air expands the primitive
alveolus during the first breath, surfactant (or its precursors stored in type
II pneumocytes) is rapidly discharged into the alveolar space (Plate 1-42).
This monomolecular layer prevents the development of an air-water interface
that other wise would have seven to 14 times as much surface tension as does
the air-surfactant interface.
According to the Laplace
equation, the pressure required to prevent collapse of a bubble caused by
surface tension is inversely proportional to the bubble’s radius. Because the
radii of primitive alveoli are very small, the collapsing forces are
correspondingly high. Therefore, as the lungs deflate, the alveolar radii are
further reduced, and the collapsing forces are proportionately increased.
Alveoli lacking surfactant thus cannot retain air after expiration, and they
collapse (Plate 1-42); infants in whom hyaline membrane disease develops have
so little air in their nonexpanded alveoli that at autopsy, the lungs
immediately sink when placed in water. Surfactant has the fortunate property of
increasing its activity as its surface area is reduced. Therefore, on
expiration, the surfactant effectively lowers the alveolar surface tension so
that air can be retained.
Without sufficient surfactant,
all breaths after the first would require great physical effort. A negative
pressure as great as 20 cm of water is required to reinflate a collapsed
primitive alveolus with a radius of 25 μm and a minimal surface tension of 50
dynes/ cm. By contrast, with surfactant present, the alveolus of a deflated lung
would have a radius of 50 μm, and its minimal surface tension would
be only 5 dynes/cm or less. Thus, a negative pressure of only 2 cm of water is
all that would be needed to maximally reinflate it under these conditions (Plate
1-42). The physical effort a premature infant lacking surfactant requires to
breathe is so great that exhaustion of the infant will soon result unless mechanical
support is provided.
Although the second breath is
much easier for a normal full-term infant, breathing is usually not completely
normal until about 40 minutes after birth. The entire lung does not become
fully inflated as soon as respiration begins, and for the first week to 10 days
after birth, small parts of the lungs may still remain underinflated.
The onset of breathing at
birth is accompanied by important and immediate circulatory system
readjustments that allow adequate blood flow through the lungs. During fetal
life, only about 12% of the cardiac output goes to the lungs because most of
the flow from the right ventricle is shunted away from the pulmonary artery to
the aorta through the large ductus arteriosus. The fluid-filled atelectatic lungs
create a high resistance in the pulmonary circulation by compressing the
blood vessels. Expansion of the lungs induces vasodilation of the pulmonary
vessels and results in a sudden increase in blood flow up to 200% or more. This
increased pulmonary blood flow, coupled with the cutting off of the large
placental circulation when the umbilical cord is tied, actually means that a
smaller quantity of blood is propelled a shorter distance within the infant.
Therefore, the most crucial event at birth is the expansion of the lungs with
the first breath of air, rather than the alterations occurring in the vascular
system. After respiration has been established, the normal vascular system is
well prepared to meet the functional demands imposed on it after birth.
 |
| SURFACTANT EFFECTS |