OVERVIEW OF CHRONIC KIDNEY
DISEASE
Chronic kidney disease (CKD) affects over 26 million adults in the
United States and is defined as 3 or more months of either (1) histopathologic
or functional evidence of kidney damage, or (2) a glomerular filtration rate
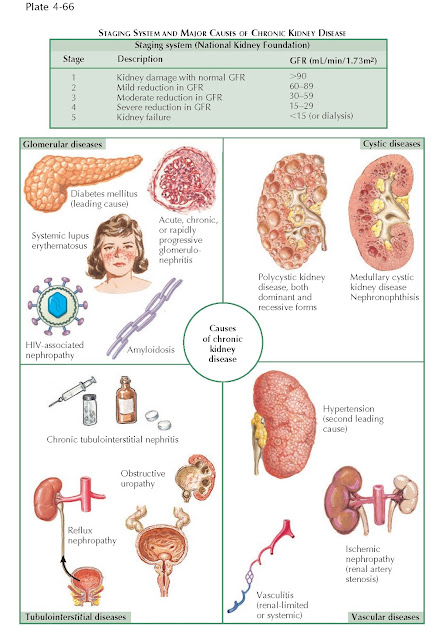
less than 60 mL/min/1.73 m2. CKD is classified into five stages based
on the degree of functional impairment, as inferred from estimations of
glomerular filtration rate.
CKD can be caused by numerous underlying processes. In
general, causes can be grouped into glomerular diseases (such as diabetic
nephropathy or lupus nephritis), vascular diseases (such as hypertension),
tubulointerstitial diseases (such as obstructive uropathy), and cystic diseases.
The most common causes are diabetes mellitus and hypertension, which together
account for over two thirds of cases.
PATHOPHYSIOLOGY
Irrespective of the primary renal disease, the kidney
initiates a compensatory response to nephron loss that is initially adaptive
but ultimately causes further loss of function. Thus the progression of CKD
depends in part on mechanisms that are independent of the inciting disease
process.
In particular, loss of a subset of nephrons results in
compensatory hyperfiltration and hypertrophy of the remaining functional
nephrons, an effect likely mediated by angiotensin II, aldosterone, endothelin,
and other hormones. The resulting intraglomerular hypertension, however,
eventually becomes maladaptive, inflicting damage to the remaining nephrons and
thereby causing a further decline in overall filtration. In the glomerulus,
podocyte (visceral epithelial cell) foot process effacement and denudation may
lead to a breakdown of the protein filtration barrier and glomerulosclerosis.
The ensuing proteinuria is thought to further promote kidney failure by
exerting toxic effects on the tubules. In addition, mesangial cells respond to
the increased pressure with proliferation and production of increased
extracellular matrix; these changes stimulate inflammation and cellular
infiltration of the mesangium and tubulointerstitium, leading to
tubulointerstitial fibrosis.
A significant number of nephrons must be lost before
these maladaptive changes are seen, and the exact quantity varies between
species and individuals. In rats, for example, one and 5/6 nephrectomy must be performed to create a model of
progressive renal insufficiency. In humans, it is well-known that donation of
one entire kidney does not generally result in loss of overall renal function. In
those individuals who do lose enough functional renal mass to experience the
maladaptive effects of hyperfiltration, the rate of further functional decline
is also variable, depending on the primary inciting factor, patient age, and
possibly genetic factors.
ASSESSMENT OF RENAL FUNCTION
The serum creatinine concentration should be used to
estimate the creatinine clearance or GFR (eGFR), which can be accomplished
using the Cockcroft-Gault formula or the modification of diet in renal disease
(MDRD) study equation, respectively.
Current guidelines recommend that serum creatinine
concentration be measured at least once per year in patients with CKD. The frequency should be increased in those with an
eGFR <60 mL/min/1.73
m2, loss of >4 mL/min/1.73 m2 per year of eGFR, or with risk factors for
progression (high level of proteinuria, hypertension, diabetes mellitus).
Patients should be referred to a nephrologist when the
eGFR falls below 30 mL/min/1.73 m2, or sooner if the primary care
physician is unable to carry out a treatment plan for CKD.
COMPLICATIONS AND MANAGEMENT
Patients with CKD are typically asymptomatic until the
disease process becomes advanced. Thus it is important to screen for CKD and
its complications in high-risk patients, such as those with diabetes mellitus
and/or hypertension, so that treatment can be initiated at an early stage.
Hypertension. Hypertension
is both a cause and consequence of progressive chronic kidney disease. It is a
cause because elevated arterial pressures are transmitted to the glomeruli of
the remaining nephrons, exacerbating hyperfiltration and accelerating further
nephron loss. It is also a consequence because, as nephron loss progresses,
there is ongoing secretion of angiotensin II and impaired excretion of excess
sodium and water. Thus more than 75% of patients with CKD suffer from
hypertension.
Blood pressure must be routinely measured in all
patients, and hypertension must be treated to slow nephron loss. Blood pressure
should be kept at 130/80 mm Hg or less, and the mainstays of treatment are
medications that block the renin-angiotensin-aldo-sterone system, including ACE
inhibitors and ARBs. These agents preferentially dilate efferent arterioles,
lowering intraglomerular pressure. Because of this partial reversal of
glomerular hyperfiltration, there is an expected and acceptable 30% increase in
serum creatinine; however, providers should carefully monitor patients for both
acute declines in eGFR and hyperkalemia.
Diuretics are often needed as well, especially as more
advanced disease leads to greater retention of sodium and water. In general, at
an eGFR <30 mL/min/1.73
m2, loop diuretics are more effective than thiazide diuretics.
Patients should also be strongly encouraged to maintain a low-salt diet.
Proteinuria. Proteinuria is
a marker of glomerular injury, but it is also understood to contribute to CKD
progression. In particular, proteins that are filtered in the glomerulus are reabsorbed in proximal tubule
cells, where they trigger inflammation, apoptosis, and fibrosis. In addition,
abnormal filtration of growth factors and cytokines also promotes tubular
injury. Thus a reduction in proteinuria has been associated with a slower
progression of chronic kidney disease, particularly among patients with
diabetic disease.
Proteinuria must be regularly assessed in all
patients. In nondiabetics, screening with urine dipstick is acceptable, but if
positive, a spot urine protein : creatinine ratio should be performed for quantification. A 24-hour urine collection
for protein can be performed; however, a spot sample is typically adequate and
is easier for the patient. In diabetics, regular screening for microalbuminuria
should be performed early in the disease course.
ACE inhibitors and ARBs have been shown to reduce
proteinuria, likely because of the reduction in intraglomerular pressure.
Studies in diabetic patients have shown that these drugs reduce proteinuria and
slow the decline of eGFR independent of their effect on
systemic blood pressure.
Bone Disease. Renal disease
also leads to numerous morphologic changes in bone, a group of phenomena
collectively known as renal osteodystrophy. This disorder encompasses a
spectrum of disease with both high bone turnover (osteitis fibrosa cystica) and
low bone turnover (adynamic bone disease and osteomalacia).
Osteitis fibrosa cystica, a high turnover disease,
reflects secondary hyperparathyroidism and is associated with bone pain and an
increased risk of fracture. High PTH levels are often seen once the eGFR
declines to less than 60 mL/min/1.73 m2 (stage 3 CKD), and they are
invariably seen when eGFR is less than 30 mL/ min/1.73 m2 (stage 4
CKD). High PTH levels initially occur because of decreased renal production of
1,25(OH)2D, the activated form of vitamin D, which results from both
a reduction in renal mass and impaired renal excretion of phosphate. The
decline in 1,25(OH)2D stimulates PTH secretion and, moreover, causes
a reduction in intestinal reabsorption of calcium, which further stimulates PTH
release. Initially, the high levels of PTH maintain serum phosphate and calcium
concentrations within normal range, at the expense of causing bone disease. As
renal dysfunction progresses, however, hyperphosphatemia ensues. In addition,
hypocalcemia eventually occurs, both because of the decline in 1,25(OH)2D
levels and because of formation of soluble calcium phosphate complexes. Skeletal resistance to PTH, which
remains chronically elevated, also appears to play a role.
In patients with stage 3-5 CKD, serum concentrations
of PTH, phosphate, and calcium should be checked regularly. According to
current guidelines, the goal PTH levels for stage 3 CKD are 35 to 70 pmol/L,
for stage 4 CKD are 70 to 110 pmol/L, and for stage 5 CKD are 150 to 300
pmol/L. Goal serum phosphate levels for stages 3 and 4 CKD are 2.7 to 4.6
mg/dL, and for stage 5 CKD are 3.5 to 5.5 mg/dL. To achieve these levels,
hyperphosphatemia is typically addressed first using a low phosphorous diet and
phosphate binders. Either calcium or noncalcium containing binders may be used,
with the choice sometimes depending on the patient’s serum calcium
concentration. Vitamin D (25-OH) levels should also be checked, and if levels
are below 30 µg/mL, supplemental ergocalciferol may be offered.
If PTH levels remain elevated despite these measures,
active vitamin D analogues (such as calcitriol) may be used in lieu of ergocalciferol; however, these agents may cause
marked elevation of serum phosphate and calcium levels, which must continue to
be carefully monitored. In patients with more advanced disease, calcimimetics
(i.e., cinacalcet) may be used, although they are only approved for those
receiving dialysis. Calcimimetics bind to the calcium sensing receptor on the
parathyroid glands, suppressing PTH release, but they are associated with an
increased risk of hypocalcemia.
If PTH levels are oversuppressed, patients can develop
adynamic bone disease, which is also associated with increased risk of
fracture. This disorder is becoming increasingly common as vitamin D analogues
are more widely used to suppress PTH. If the PTH level falls below 100 pmol/L,
the risk of adynamic bone disease is high, and dosages of vitamin D analogues
and calcium-based phosphate binders should be reduced.
Finally, osteomalacia, another form of low bone
turn-over disease, can be seen in some patients due to vitamin D deficiency or aluminum toxicity. With the near elimination of
aluminum-based binders in clinical practice, however, aluminum toxicity is now
uncommon.
The specific type of bone disease can be definitively
diagnosed with bone biopsy, which is not routinely performed in clinical
practice. Instead, the presence of bone disease is typically inferred from
abnormal PTH levels.
Acidosis. Once GFR
declines to 40 to 50 mL/ min/1.73 m2, patients are not able to
excrete their daily acid load. The remaining functioning nephrons have
maximized their ammonium excretion, and excretion of titratable acids may also
be reduced because of the dietary restriction of phosphate and use of phosphate
binders. The result is metabolic acidosis with a positive anion gap, which is
usually discovered as a low serum bicarbonate level on a routine assessment of
serum chemistries. Current guidelines recommend that serum bicarbonate be
checked annually in patients with stage 3 CKD and every 3 months in patients
with stage 4 or 5 CKD. It is
recommended that serum bicarbonate concentrations be maintained at 22 mEq/L or
greater. Oral bicarbonate replacement can be used to achieve this goal.
Anemia. CKD leads to
normocytic anemia due to inadequate renal production of erythropoietin. Anemia
is sometimes seen in stage 3 CKD and is almost always seen in stage 4 CKD.
Current guidelines recommend that hemoglobin levels be measured annually in any
patient with CKD.
In males with hemoglobin less than 13 g/dL and females
with hemoglobin less than 12 g/dL, further workup should be performed,
including a complete blood count, reticulocyte count, and an assessment of iron
stores. Relative iron deficiency is common and contributes to the decreased production
of red cells. One reason for iron deficiency is that the inflammatory cytokines
released in CKD promote secretion of hepcidin, which blocks iron absorption
from the GI tract and iron release from macrophages.
In general, erythropoiesis-stimulating agents are used
to maintain a hemoglobin level of 11 to 12 g/dL. The target should not exceed
13 g/dL. In patients receiving this treatment, iron stores should be assessed
and replenished as needed to avoid apparent erythropoietin resistance. Oral
iron supplements, such as iron sulfate or iron gluconate, are commonly given.
If patients are resistant to these supplements because of impaired intestinal
absorption, intravenous iron preparations may be used instead.
Cardiovascular Disease. Cardiovascular disease is the leading cause of death among patients with
chronic kidney disease, and it affects 40% of dialysis patients compared with
10% of the general population. Patients with CKD are more likely to have
classic risk factors for cardiovascular disease, such as hypertension, diabetes mellitus, and
hyperlipidemia. CKD itself, however, also appears to be an independent risk
factor for CVD, and recent studies have shown a strong correlation between
declines in eGFR and increased cardiovascular events.
Numerous factors are responsible for this association.
Vascular calcification appears to result from the use of calcium-based phosphate
binders and vitamin D analogues. It may affect the intimal layer, leading to atherosclerotic
plaques, and/or the medial layer, leading to vessel stiffening. The inflammation
and secondary hypertension associated with CKD also accelerate vascular
disease.
In addition to increasing the risk for cardiovascular
disease, CKD also increases the risk of left ventricular hypertrophy by causing
hypertension, anemia, and hypervolemia. The prevalence of left ventricular hypertrophy is much higher among dialysis patients than the general
population.
Modification of cardiovascular risk factors such as smoking, hyperlipidemia, and hypertension should remain a primary focus of therapy. Even after
kidney disease becomes more advanced, many patients die from cardiovascular
disease before ever reaching end stage renal disease.
Hyperkalemia. Renal
excretion of potassium does not become significantly impaired until stage 4 CKD;
however, a potassium rich diet or use of ACE inhibitors/ARBs can lead to hyperkalemia at
earlier stages.
A low potassium diet (<50 mEq/day) may be instituted as a
preventive measure. In addition, treating metabolic acidosis will lower serum
potassium levels, and loop diuretics may be used to promote urinary excretion
of potassium. Dialysis may be required if hyperkalemia becomes refractory to
medical management.
Volume Overload. Overt
symptoms of volume overload, such as peripheral and pulmonary edema, do not
typically occur until stage 5 CKD but can be precipitated in earlier stages by
increased salt intake or coexisting congestive heart failure. These symptoms
can generally be treated with sodium restriction and additional diuretics.
Uremia. As renal
dysfunction becomes very advanced, the retention of toxic substances in the
general circulation can lead to numerous abnormalities that are together known
as uremia. In general, the term is used to describe the effects of those retained
toxins that have not been identified or are poorly understood. Signs and
symptoms of uremia include loss of appetite, weight loss, fatigue, altered
mental status, peripheral neuropathies, nausea, vomiting, pruritus, and
platelet dysfunction. Although uremia is associated with an elevated blood urea
nitrogen (BUN) concentration, BUN itself is not felt to be the cause of uremia.
ADDITIONAL CONSIDERATIONS
It is essential that renally cleared medications be
dosed based on eGFR. Furthermore, drugs that may precipitate an acute decline
in renal function, such as nonsteroidal antiinflammatory drugs should be
avoided. Careful consideration must be given when administering iodinated
contrast due to the risk of acute renal failure. In addition, gadolinium-based
contrast should be used judiciously in patients with stage 4 or 5 CKD because
of the increased risk of nephrogenic systemic fibrosis.
END STAGE RENAL DISEASE
Ultimately, a small proportion of patients with CKD will progress to end-stage renal disease (ESRD),
defined as the need
for dialysis therapy or kidney transplantation. The rate of progression,
however, is highly variable across patients.
Dialysis is typically initiated when the eGFR falls
below 10 mL/min/1.73 m2; however, there are numerous acute indications
as well, which include refractory hyperkalemia, volume overload, and uremia.
In most patients, preemptive kidney transplantation is
preferred over ongoing dialysis because the long-term survival is significantly better. Compared with dialysis patients
on the transplant list, patients who receive a kidney transplant have an
initial increase in mortality; however, at 4 months post-transplant the risk of
death is equal between the two groups, and thereafter transplanted patients
have a 68% lower risk of mortality compared with patients on dialysis. The
survival benefit is particularly robust among patients with diabetes.