TESTICULAR TUMORS II: TERATOMA,
CHORIOCARCINOMA, IN SITU NEOPLASIA
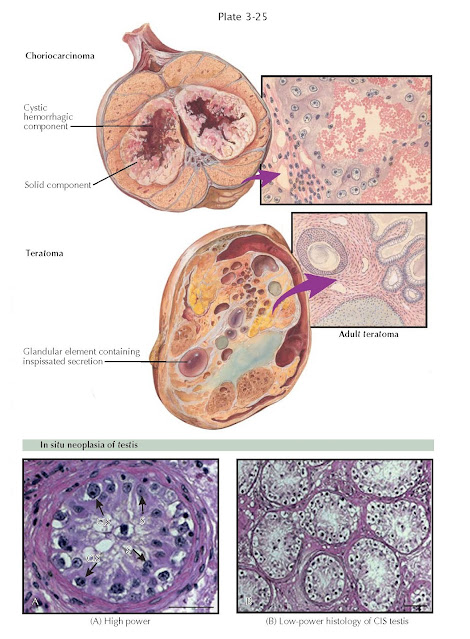
Teratomas (Greek: monstrous tumor) are encapsulated tumors with tissue or organ components resembling normal derivatives of all three germ layers but foreign to the location in which they are found. Teratomas have been reported to contain hair, teeth, bone, and very rarely more complex organs such as eye, torso, and hands and feet. Teratomas comprise about 5% of testicular tumors and are generally classified as malignant. They occur in several forms, including mature and immature teratomas and dermoid cysts, the latter of which contains developmentally mature skin (ectoderm) and are considered benign.
Teratomas show a remarkably
wide variation in gross and microscopic appearance. Epithelial components of
the tumors tend to reproduce glandular tissue, cysts,
cartilage, joints, skin, teeth, neuroepithelium, epidermoid cysts, enteric
glands, smooth muscle, salivary glands, respiratory epithelium, lymphoid
tissue, transitional epithelium, and even cardiac muscle and bone. Teratomas
may be so poorly differentiated that only unrecognizable structures are
present. Pure teratomas do not increase fetoprotein or human chorionic
gonadotropin (hCG) levels. The prognosis for teratoma, if it contains no
malignant focus of leukemia, sarcoma, or carcinoma, is favorable because of the
infrequent occurrence of metastases. In the testis, teratomas are most commonly
found in association with other nonseminomatous germ cell tumor types and are
malignant. When found in association with embryonal carcinoma or choriocarcinoma,
they are called terato- carcinomas. Pure teratomas are relatively chemo- and
radioresistant and are treated by radical orchiectomy and surgical extirpation
in general.
Especially in mixed germ
cell tumors, the histology of the primary tumor may not conform to that of
metastases, which may contain any combination of the primary tumor cell types.
For example, adult teratomas may metastasize as teratomas or as embryonal
carcinomas. It may be that relatively undifferentiated malignant primary tumor
cells undergo a maturation process during metastasis.
Choriocarcinomas are among
the most highly malignant tumors in the body. This fast-growing tumor develops
from trophoblastic cells that form the placenta and help embryos attach to the
uterus. They are rare (2% of testis tumors) and most commonly observed in
conjunction with embryonal carcinomas and teratocarcinomas. They uniformly
secrete hCG into the serum. Grossly, they appear remarkably hemorrhagic and
necrotic. Histologically, they consist of giant syncytiotrophoblastic cells
with large, atypical nuclei intermingled with cytotrophoblasts, surrounding
blood spaces, similar in structure to placental chorionic villi. Aggressive
treatment with surgical resection and cisplatin based
chemotherapy has resulted in high cure rates.
Testicular germ cell cancers
may begin in a noninvasive form called carcinoma in situ (CIS) or intratubular
germ cell neoplasia. CIS may not always progress to invasive cancer and it is
estimated that it takes 5 years for CIS to progress to an invasive form of germ
cell cancer. It is found in the contralateral testis in 5% of testis cancer
cases. In addition, it is currently thought that CIS is the common precursor to
all testis germ cell tumor types (except for juvenile yolk sac and teratoma
tumors and spermatocytic seminomas). The assumption that CIS is the precursor of germ cell tumors is supported by the
frequent observation of CIS in testis tissue surrounding invasive cancer and
the development of invasive germ cell cancers in patients in whom CIS has been
diagnosed. CIS causes no symptoms and has few findings, as it is generally found
on testis biopsy. CIS cells are large with distinct nucleoli and are located in
a single row at the usually thickened basement membrane of seminiferous tubules.
Testicular tissue with CIS is frequently atrophic and may be dysgenetic, with poorly differentiated tubules, poor spermatogenesis, and
microcalcifications. The most common marker for CIS is placental alkaline
phosphatase (PLAP), a tissue
specific alkaline
phosphatase. The best treatment for CIS is controversial, since CIS does not
always become invasive cancer. However, if treatment is chosen, localized
low-dose radiotherapy (14 to 16 Gy) to the testis eradicates CIS and germ cells
(and therefore fertility) while maintaining Le dig cell function and androgen balance in most men.