DIAGNOSTIC CORONARY ANGIOGRAPHY
The right coronary artery (RCA) arises from the right coronary sinus and runs in the right atrioventricular (AV) groove (Fig. 12.1). The conus artery is typically the first branch that arises from the RCA and supplies the right ventricular outflow tract. The sinoatrial nodal and AV nodal branches also arise from the RCA and supply the sinus node and the AV node, respectively. Marginal branches usually arise from the mid-RCA and supply the right ventricular wall. The distal RCA gives rise to right posterolateral branches and the posterior descending artery (PDA) in 85% of cases (defined as right dominance). The PDA arises from the left circumflex (LCX) in 8% of cases (defined as left dominance), and from both the RCA and LCX in 7% of cases (defined as co-dominance). The PDA runs in the posterior interventricular groove and supplies the posterior aspect of the interventricular septum.
 |
FIG
12.1 Coronary arteries and cardiac
veins.
The left main coronary artery arises from the left coronary sinus and bifurcates into the left anterior descending (LAD) and LCX arteries (Fig. 12.1). In a minority of cases, the left main coronary artery trifurcates into the LAD artery, ramus intermedius artery, and LCX artery. The LAD artery runs in the anterior interventricular groove toward the apex of the heart and supplies the anterior wall of the left ventricle. Septal perforator branches arise from the LAD artery and supply the interventricular septum. Diagonal branches also arise from the LAD artery and supply the anterolateral wall of the left ventricle. The LCX artery runs in the left AV groove and provides obtuse marginal branches that supply the posterolateral wall of the left ventricle. As noted previously, in a minority of cases, the PDA will arise from the LCX artery.
There are many schemes for describing coronary anatomy: the Coro- nary
Artery Surgery Study (CASS) classification, the Synergy Between PCI With Taxus
and Cardiac Surgery (SYNTAX) classification, and the Bypass Angioplasty Revascularization Investigation (BARI)
modification of the CASS map are some of the most widely accepted.
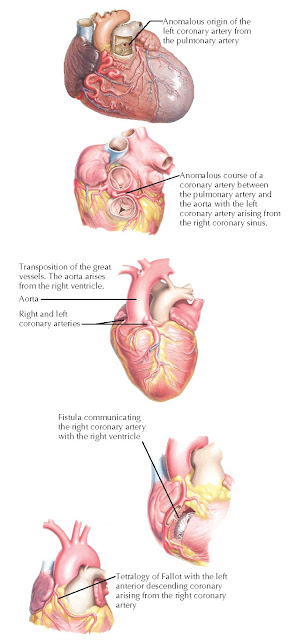
CORONARY ARTERY ANOMALIES
Coronary artery anomalies are typically a result of abnormal
embryological development and are found in 1% to 1.5% of cases. Most coronary
artery anomalies are clinically benign. The most common coronary artery anomaly
is the presence of separate origins of the LAD and LCX arteries, which occurs
in 0.4% to 1% of cases and may be associated with a bicuspid aortic valve.
Clinically significant anomalies include a coronary artery originating from the
opposite coronary sinus (e.g., left main coronary artery originating from the
right coronary sinus), the presence of a single coronary ostium leading to a
single coronary artery, a coronary artery coursing between the great vessels
(e.g., between the aorta and pulmonary artery), and a coronary artery leading
to decreased oxygenation of the myocardium (e.g., a coronary artery originating
from the pulmonary artery or a coronary artery–ventricular fistula) (Fig. 12.2).
FIG
12.2 Congenital coronary artery
anomalies.
PREPROCEDURAL EVALUATION
Obtaining a history, physical examination, routine laboratory data (such
as chemistry, complete blood count, and coagulation studies), a 12-lead ECG,
and a transthoracic echocardiogram can provide valuable information for procedural
planning. An accurate history can help determine patient candidacy for PCI and
dual antiplatelet therapy in the event that the angiographic findings
demonstrate obstructive CAD. Physical examination of the peripheral pulses can
help plan the site of vascular access. Stress testing can be performed to risk
stratify patients before coronary angiography and to help localize the area of
myocardial ischemia.
Indications
The American College of Cardiology and American Heart Association have
published guidelines on the indications for diagnostic coronary angiography
(Table 12.1). Patients with acute coronary syndromes should undergo emergent or
urgent diagnostic coronary angiography. In particular, patients with
ST-elevation myocardial infarction (STEMI) should undergo emergent coronary
angiography with the goal of establishing reperfusion with angioplasty within
90 minutes of clinical presentation. Patients who have a non-STEMI or unstable
angina, and who are at intermediate or high risk for adverse events should
undergo early coronary angiography within 24 to 72 hours. Patients with
high-risk features (e.g., refractory angina, hemodynamic or electrical
instability, or cardiogenic shock) should undergo coronary angiography within the first few hours of clinical
presentation.
Patients with stable angina and certain clinical features may undergo coronary angiography without previous stress testing. For instance, patients who have symptoms highly typical of angina or patients who have symptoms with minimal or no exertion should be directly referred for coronary angiography without previous stress testing. Additional clinical features that may prompt referral for coronary angiography without previous stress testing include a history of myocardial infarction, previous percutaneous or surgical revascularization, and congestive heart failure.
The only absolute contraindication to diagnostic coronary angiography is
the lack of patient consent. There are many relative contraindications that can
increase the risks of the procedure in patients with certain co-morbidities (Table 12.2). For example, acute
renal failure or preexisting renal dysfunction, especially in patients with
diabetes mellitus, can increase the risk of contrast-induced nephropathy.
Electrolyte abnormalities and/or digitalis toxicity can increase the risk of
arrhythmias during contrast injection. Active bleeding, severe
thrombocytopenia, and/or severe coagulopathy from co-morbidities or medications
(e.g., such as warfarin or the new anticoagulants) can increase the risk of
vascular complications and/or bleeding risk in the setting of PCI.
Decompensated heart failure, severe aortic stenosis, or uncontrolled
hypertension can increase the risk of acute flash pulmonary edema and
respiratory failure when the patient is required to remain supine during the
procedure. Other relative contraindications include active infection, allergic
reactions to iodinated contrast agents, severe peripheral vascular disease, pregnancy, and patient inability
to cooperate.
PROCEDURAL TECHNIQUE
Diagnostic coronary angiography is routinely performed in a cardiac
catheterization laboratory. Written informed consent must be obtained before
the procedure. Patients should be informed of the indication, benefits, risks,
and alternatives of coronary angiography. If there is a possibility of
obstructive CAD that leads to PCI, patients should also be informed of the
indication, benefits, risks, and alternatives of PCI before the procedure. Once
in the cardiac catheterization laboratory, patients are prepared with
antiseptic solution at the access site and draped in a sterile fashion. A
time-out is performed with the physicians, nurses, and cardiovascular
technologists to verify the patient, procedure, indication, access site, and
any allergies. Sedatives with analgesics are then admin-istered intravenously
for conscious sedation.
Arterial Access
Arterial access can be obtained via percutaneous puncture of the common
femoral artery (CFA), brachial artery, or radial artery (Fig. 12.3). Although
the femoral approach is historically the most commonly used site for arterial
access, the radial approach has become increasingly popular and may be the
preferred strategy in patients with morbid obesity, decompensated heart
failure, or severe peripheral artery disease.
 |
FIG
12.3 Left-sided heart
catheterization.
The femoral approach requires the proper identification of anatomic landmarks before vessel puncture (Fig. 12.4). The fluoroscopic landmark for the optimal CFA entry site is generally considered to be the upper one-half or the upper one-third of the femoral head. In a study of 200 femoral angiograms, the CFA bifurcated below the center of the femoral head in 98% of patients, whereas in another study of 208 femoral angiograms, the bifurcation of the CFA occurred below the upper one- third of the femoral head in 99% of patients. The access site can be confirmed under fluoroscopy and/or vascular ultrasound can be used. Arterial puncture above the inguinal ligament may lead to an increased risk of bleeding complications (e.g., a hematoma or retroperitoneal bleeding). Risk factors for vascular complications are both clinical and anatomic, and include age, female sex, weight, uncontrolled hypertension, previous arteriotomy at the same site, type and level of anticoagulation, arterial sheath size, renal failure, concomitant venous sheath, peripheral vascular disease, prolonged sheath duration, and location of the arteriotomy. Arterial puncture below the femoral head may lead to an increased risk of vascular complications (e.g., an arteriovenous fistula, a pseudoaneurysm, or a hematoma).
 |
| FIG 12.5 Radial artery access. |
The hand receives dual circulation from the radial artery and the ulnar
artery through the superficial and deep palmar arches (Fig. 12.5). The Allen
test assesses the patency of the arch circulation and involves the simultaneous
compression of the radial and ulnar arteries at the level of the wrist for 1 to
2 minutes, which leads to pallor of the hand, followed by the release of ulnar
compression. The spontaneous return of color to the hand in 5 to 10 seconds
indicates patency of the palmar arch. Although theoretically attractive, the
Allen test is less widely used now than previously because it has not been
shown to correlate with outcomes. The optimal access site for the radial
approach is the point of radial artery pulsation, palpated approximately 2 cm
proximal to the radial styloid process. Vasospasm is a possible vascular
complication of the radial approach because the radial artery is a
small-caliber vessel with a relatively large muscular media. To prevent
vasospasm, a vasodilator such as verapamil is routinely administered
intra-arterially through the sidearm of the sheath after obtaining radial
access.
Coronary Artery Cannulation
A standard 0.035-inch, J-tipped guidewire is introduced through the
sheath in the access artery and advanced to the ascending aorta. The guidewire
is used to guide the coronary catheter to the aortic root, and is always
advanced before the catheter to prevent the proximal edges of the catheter from
causing vascular damage. Once the proximal end of the catheter is positioned in
the root, the guidewire is removed from the catheter and the catheter is
connected to the manifold. When the pressure transducer connected to the
manifold confirms an appropriate aortic pressure waveform, the catheter is
flushed with heparinized saline and loaded with contrast. Using fluoroscopy,
the catheter is advanced or withdrawn as it is being rotated until it engages
the ostium of the coronary artery. During coronary artery cannulation, the
catheter location and pressure tracing should be carefully monitored to ensure
that the catheter tip is coaxial with the ostium of the coronary artery and is
not either against the arterial wall or obstructing flow in the artery.
Coronary angiography is performed with cineradiography during injection of
contrast. The left and right coronary arteries are typically cannulated by
different catheters (Figs. 12.6 and 12.7). Catheter selection depends on the
access site, the coronary artery being investigated, the location of the
coronary ostium, the diameter of the aortic root, and operator preference.
 |
| FIG 12.6 Coronary arteries: angiographic views. AV, Atrioventricular; SA, sinoatrial. |
In patients with a history of CABG, all bypass conduits should be
investigated. Saphenous vein grafts are anastomosed to the anterior wall of the
ascending aorta above the sinuses of Valsalva. Several strategies can be used
to assist with graft cannulation, including a review of previous postsurgical
angiograms, identification of markers that were placed during surgery at the
ostium of the saphenous vein grafts, and use of different views (left anterior
oblique [LAO] to cannulate grafts to the RCA and right anterior oblique [RAO]
to cannulate grafts to the left coronary system). The left internal mammary
artery (LIMA) is routinely used in surgical revascularization and typically
arises anteriorly from the left subclavian artery, several centimeters distal
to the vertebral artery (Fig. 12.8). A 0.035-inch guidewire is advanced through
a catheter into the distal left subclavian artery either when the catheter is
in the aorta or after it has been used to cannulate the left subclavian artery.
The catheter is advanced into the
subclavian artery and then slowly withdrawn
with gentle counter clock wise rotation (so that the catheter tip faces anteriorly) until it cannulates
the LIMA.
 |
FIG 12.8 Left internal mammary artery (LIMA) and subclavian disease. LAD, Left anterior descending. |
Comprehensive evaluation of the coronary arteries requires angiography in
multiple views to ensure that all vessel segments are visualized without foreshortening or overlap (see Fig. 12.6).
Rotating the image intensifier to different positions around the patient allows
images to be obtained in different views. Angiographic views consist of a
specific projection (e.g., RAO, LAO, or anteroposterior [AP]), with a specific
angulation toward the head or foot of the patient (designated as cranial or
caudal, respectively). The most
common views for RCA angiography include the RAO projection, LAO projection,
and AP projection with cranial angulation (see Figs. 12.6 and 12.7). The most
common views for left coronary angiography include the RAO projection with
cranial and caudal angulation, the LAO projection with cranial and caudal
angulation, and the AP projection with cranial and caudal angulation.
Angiographic Analysis
The essential components of coronary angiographic analysis are listed in Table 12.3. The origin, caliber, course,
and branches of all major coronary
arteries should be identified. The presence, location, severity, and appearance
(e.g., eccentricity or calcification) of any atherosclerotic plaque in the
major coronary arteries should be described. The severity of luminal narrowing
can be quantified by comparing the minimal diameter of the narrowed coronary
segment with that of an adjacent normal-appearing reference segment. Although
experienced observers are able to visually estimate the degree of stenosis, the
severity of the stenosis can be quantified using calipers or quantitative
computer angiography. The flow in coronary arteries can be defined using the
Thrombolysis In Myocardial Infarction (TIMI) flow grading scale. TIMI 3 flow describes normal flow with complete
filling of the distal vessel. TIMI 2 flow describes delayed or sluggish flow
with complete filling of the distal vessel. TIMI 1 flow describes faint flow
beyond the stenosis with incomplete filling of the distal vessel. TIMI 0 flow
describes a completely occluded artery with no distal flow beyond the lesion.
The presence of anomalous arteries, myocardial bridging, fistulas, dissections, aneurysms, and spasm should
also be noted. In patients with a history of CABG, graft patency and the
presence of competitive flow should be observed. In the setting of total
occlusion of a coronary artery, prolonged cineradiography allows the capture of
late-filling collateral circulation that may exist (Fig. 12.7). The collaterals
can either originate from the occluded artery or a different coronary artery or bypass graft.
LIMITATIONS
Coronary angiography has several limitations in the evaluation of CAD.
First, it produces a two-dimensional representation of three-dimensional
coronary anatomy. As such, the severity of CAD may be underestimated. Second,
coronary angiography delineates the vessel lumen but is unable to provide
accurate information about the vessel wall. Angiographic findings of normal
vessel lumen cannot exclude underlying disease of the coronary endothelium. Furthermore, proper interpretation of
stenosis severity involves identification of an appropriate reference segment
with which to compare the diseased segment; this may prove to be difficult
because of the possibility of inaccurate vessel wall delineation.
These limitations have led to advances in technology that can supplement
coronary angiographic analysis. Fractional flow reserve (FFR) uses a pressure
wire (coronary guidewire attached to a pressure transducer) to measure the
intracoronary pressure distal to a stenosis, and compares this distal
intracoronary pressure with aortic pressure at rest and during maximal coronary
hyperemia (see Chapter 26). FFR is calculated based on this comparison, can
help determine the hemodynamic significance of a lesion, and is clinically
useful in the assessment of an angiographically intermediate lesion.
Intravascular ultrasound (IVUS) uses a catheter with an ultrasound core to
provide cross-sectional images in which the three layers (intima, media, and
adventitia) of the vessel can be identified and characterized (Fig.12.9). Optical coherence tomography (OCT) also uses
an intracoronary catheter, but with an optical imaging core that provides
high-resolution cross-sectional images. OCT and IVUS can be used to assess the
size of the artery, the vascular wall and plaque composition, and burden, and
can be used to assess and optimize PCI results.
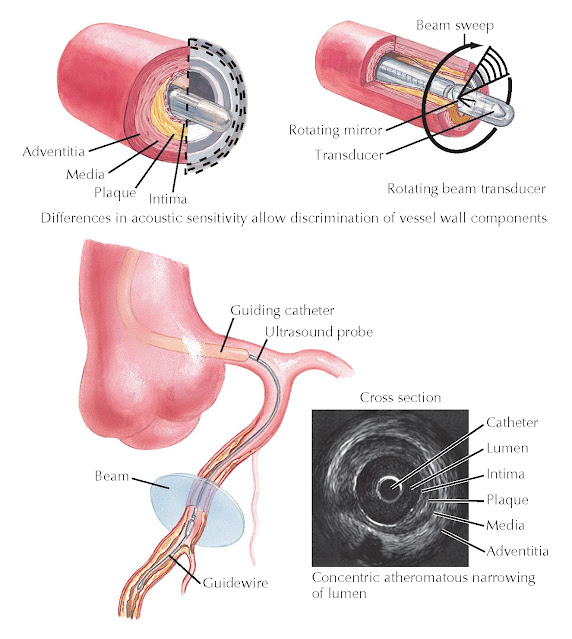
FIG 12.9 Intravascular ultrasound.
The major complications that can occur during or immediately after
coronary angiography include death, myocardial infarction, and stroke. The risk
of major complications is 0.3%. Minor complications include coronary artery dissection, bleeding, vascular
complications, arrhythmias, and contrast reactions. The risk of any of these
complications is still <2%. Patient comorbidities that increase the risk of
complications include acute coronary syndrome, left main CAD, shock, congestive
heart failure, severe valvular disease, renal failure, peripheral vascular
disease, increased age, and previous anaphylactoid reaction to contrast media.
Complication rates from coronary angiography have remained remarkably
consistent across registries from the 1980s. Complication rates from PCI have been
the focus of more recent registry analysis.