LEFT HEART CATHETERIZATION
Left heart catheterization is distinct from coronary angiography, which involves the cannulation and interrogation of the coronary arteries. Patients who undergo coronary angiography or right heart catheterization typically also undergo left heart catheterization as part of a comprehensive hemodynamic evaluation. The common indications for left heart catheterization include the evaluation of LV hemodynamics, LV systolic function, cardiomyopathy, valvular disease (e.g., aortic stenosis or mitral regurgitation), and intracardiac shunts (e.g., ventricular septal defects). The absolute contraindications for left heart catheterization include patient refusal, known or suspected LV thrombus, and mechanical prosthetic aortic valves. The relative contraindications for left heart catheterization include active bleeding, severe thrombocytopenia, severe coagulopathy, active infection, severe peripheral vascular disease, pregnancy, and patient inability to cooperate.
Procedural Technique and Data Interpretation
Left heart catheterization is routinely performed in the cardiac catheterization
laboratory. Arterial access is obtained via percutaneous puncture of the common
femoral artery, brachial artery, or the radial artery, as described in Chapter
18. A standard 0.035-inch, J-tipped guidewire is introduced into the access
artery and is used to guide the catheter to the ascending aorta. The most
common catheters that are used in left heart catheterization are the pigtail
catheter and the Judkins right (JR) catheter. Each catheter requires a specific
technique to cross the aortic valve and enter the LV. A pigtail catheter is
rotated to make the pigtail resemble a “6” and is gently advanced until it
pushes against the aortic valve and prolapses into the LV. When the JR catheter
is used, the catheter is advanced several centimeters above the aortic valve
and rotated so that the distal curve points between 4:00 and 6:00 o’clock. The
guidewire is then advanced across the aortic valve, and the JR catheter is advanced into the LV over the
wire. The typical radiographic view
for crossing the aortic valve is the right anterior oblique (RAO) projection.
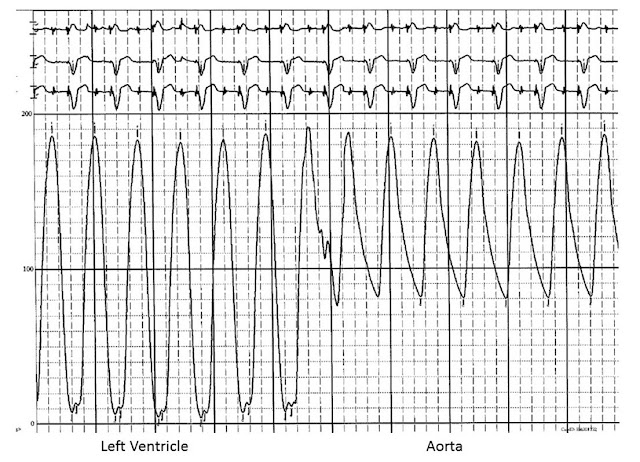 |
| FIG 13.2 Pressure tracing during catheter pullback from the left ventricle to the aorta |
Once the catheter has crossed the aortic valve, the distal end of the
catheter is positioned in the mid-cavity of the LV. The guidewire is removed,
and the catheter is connected to a manifold with a pressure transducer. The LV
pressure waveform is carefully examined and recorded. In particular, the LV
peak systolic pressure and the LV end-diastolic pressure are noted (Fig. 13.2).
Right heart catheterization with simultaneous recording of right-sided
pressures can help further define specific hemodynamic profiles (Table 13.1).
Left heart catheterization is also useful in determining the presence and
etiology of a LV outflow tract pressure gradient. A pressure difference between
the LV apex and the aorta can be caused by a fixed obstruction at the
subvalvular, valvular, or supravalvular level; or by a dynamic obstruction of
the LV outflow tract in patients with hypertrophic obstructive cardiomyopathy (Fig.
13.3). A pressure gradient can be measured by several methods: a “pullback”
across the aortic valve in which the catheter is slowly retracted from the LV
into the aorta; a recording of simultaneous LV and femoral arterial pressure
(used as a surrogate for aortic pressure); and a recording of simultaneous LV
and aortic pressure (with a dual-lumen catheter with one lumen in the LV and
the other lumen in the aorta). In all of these methods, the location of the
obstruction can be estimated by slowly retracting an end-hole catheter from the
LV apex and noting where the pressure decreases. Dynamic LV outflow tract
obstruction, which can occur in the setting of massive septal hypertrophy with
or without systolic anterior motion of the mitral valve, can be provoked by
means of various maneuvers that decrease either preload and/or
afterload (e.g., Valsalva maneuver or administration of nitroglycerin), or that
increase contractility (e.g., isoproterenol infusion or inducing a premature
ventricular contraction).
 |
FIG 13.3 left ventricular apex and aorta in (A) aortic stenosis and (B)
hypertrophic obstructive cardiomyopathy. In this patient with aortic
stenosis, there is an approximate 40 mm Hg pressure change across the aortic
valve. In the patient with hypertrophic obstructive cardiomyopathy, there is
minimal pressure difference at baseline. After a premature ventricular
contraction, the left ventricular systolic pressure exceeds aortic systolic
pressure by >100 mm Hg. In the first sinus rhythm beat after a premature
ventricular contraction, there is a decrease in aortic pulse pressure as
compared with the last sinus beat before the premature ventricular contraction.
This is known as the Brockenbrough-Braunwald-Morrow sign.
 |
FIG 13.4 Measurement
of Left Ventricular Function With Ventriculography. LAO, Left anterior oblique; RAO, right anterior oblique.
After hemodynamic assessment, left ventriculography can be performed to estimate the LV ejection fraction, examine function of specific LV walls, measure the presence and severity of mitral regurgitation, and identify any ventricular septal defects. Left ventriculography is performed with cineradiography, and simultaneous power or manual injection of contrast. The typical angiographic views for left ventriculography are the RAO and the left anterior oblique (LAO) projections (Fig. 13.4). The RAO projection provides the best visualization of the inferior, apical, and anterior walls. The LAO projection provides the best visualization of the septal, lateral, and posterior walls, as well as the LV outflow tract and the aortic root. By convention, mitral valve regurgitation is quantified by observing the degree of opacification of the left atrium relative to the LV (Fig. 13.5). Mitral regurgitation is graded as follows:
1+: Contrast does not opacify the entire LA and clears with every
heartbeat.
2+: The entire LA is faintly opacified to a degree less than that of the
LV after several beats, and it is not cleared by a single beat.
3+: The LA is completely opacified, and the degree of opacification
equals that of the LV.
4+: The LA is completely opacified in a single beat, and the opacification
increases with each beat. In addition, in 4+ mitral regurgitation, contrast can be seen filling the pulmonary
veins.


![(A) Contrast injection into the left ventricle in a patient with severe mitral regurgitation (right anterior oblique [RAO] projection; note opacification of the left atrium and pulmonary veins). (B) Contrast injection into the left ventricle in a patient with a ventricular septal defect (left anterior oblique projection [LAO]; note that the right ventricle is opacified). (A) Contrast injection into the left ventricle in a patient with severe mitral regurgitation (right anterior oblique [RAO] projection; note opacification of the left atrium and pulmonary veins). (B) Contrast injection into the left ventricle in a patient with a ventricular septal defect (left anterior oblique projection [LAO]; note that the right ventricle is opacified).](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEjf9w8Jmf7qj2ReImP7aQxXeu_WZ4dWCNqyrK_0EpNVDWOt9tJSmT03KNVzuiSgnDQeYbdXQ4a25iOjf13pyqobZBG4QiTCna8yqqnc30_dr8U7TBNBUp4T7l6J9E9_b8GIw7VwN4UVv4_k/w640-h518/Cardiology_Page_121.jpg)



