PULMONARY EDEMA
Gas exchange occurs at the delicate interface between air and blood consisting of the alveolar epithelium and capillary endothelium. Flooding of the interstitium and alveoli with fluid and solutes from the pulmonary microvascular space disrupts this interface and is an important cause of dyspnea, hypoxemia, and respiratory failure. The pathophysiologic mechanisms that cause pulmonary edema differ among the conditions that can lead to this problem. Understanding these mechanisms provides a rationale for management (see Plate 4-127).

PULMONARY EDEMA: PATHWAY OF NORMAL PULMONARY FLUID RESORPTION
NORMAL PHYSIOLOGY
The familiar Starling relationship applies to the
pulmonary microvasculature as it does in other capillary beds and estimates the
net fluid flux (Q) across the capillary membrane from the microvascular space
(mv) into the perimicrovascular interstitial fluid (if). The important variables
are the total surface area of the microvasculature (S), the vascular
permeability per unit surface area (L), and the net hydrostatic pressures
across this membrane (Pmv - Pif ), offset partially by the plasma colloid oncotic
pressure within the microvasculature as opposed to the somewhat lower colloid
osmotic pressure in the interstitium (mv -if ). The difference in osmotic pressures across the
pulmonary capillaries is less than in other capillary beds, and low albumin
states alone do not cause
pulmonary edema.
In the normal lung, the tight junctions of the
alveolar epithelium prevent fluid from entering the alveoli, so that the fluid
transudate enters the perimicrovascular interstitial space and then drains
proximally through the pulmonary lymphatics into the venous system. The two
most common perturbations that overwhelm this homeostasis are an elevation in
capillary hydrostatic pressure and an increase in the permeability of the
microvasculature (see Plate 4-128).
CARDIOGENIC PULMONARY EDEMA
Pulmonary edema from increased hydrostatic pressures
is almost always caused by increased left atrial filling pressures from cardiac
dysfunction or volume overload and is termed cardiogenic pulmonary edema.
Common clinical situations are acute coronary syndromes, systolic or diastolic
heart failure, valvular heart disease, and volume overload from acute or
chronic renal failure. Because the permeability of the capillaries to proteins
is preserved, the fluid in the alveoli is low in protein. Management is focused
on reducing the filling pressures with diuresis and afterload reduction, as well
as specific therapies for the underlying disorder (e.g., coronary
revascularization, valvular surgery, renal replacement therapy).
NONCARDIOGENIC PULMONARY EDEMA
Pulmonary edema may occur even with normal hydrostatic pressures if there is an increase in the permeability of the endothelial
and epithelial membranes. As both proteins and fluids leak through these altered membranes, the amount of protein in
the edema fluid is elevated. The most frequent cause of noncardiogenic pulmonary
edema is acute lung injury (ALI) initiated by inhaled or ingested toxins or by
inflammatory mediators released in response to pulmonary or systemic insults.
ALI and adult respiratory distress syndrome (ARDS) are most frequently
associated with pneumonia, aspiration of gastric contents, sepsis syndromes,
pancreatitis, major trauma, and multiple blood transfusions.
The management of patients with ALI and ARDS is
definitive treatment of the underlying disorder and supportive care during resolution of
the lung injury. Despite the severity of the lung injury, most patients with
ARDS do not die from respiratory failure but instead from the underlying
illness or from complications of the complex supportive care. Ventilatory
strategies for patients with ARDS now use low tidal volumes (6 mL/kg ideal body
weight) so as not to damage the remaining aerated alveoli with excessive
distending pressures or volumes. Noncardiogenic pulmonary edema can also be
worsened by an increase in hydrostatic pressures from sepsis-associated cardiac
dysfunction or overly aggressive volume resuscitation.
SPECIFIC
CLINICAL CAUSES OF NONCARDIOGENIC
PULMONARY EDEMA
High-altitude pulmonary edema usually occurs in
individuals ascending to altitudes above 3000 m (9000 ft) above sea level even
if they are athletically fit. Current evidence suggests that some individuals
have accentuated pulmonary vasoconstriction in response to hypoxemia, perhaps
from impaired nitric oxide production or exaggerated sympathetic responses,
causing high pulmonary artery pressures that tear or fracture the pulmonary
capillaries. This can be fatal unless managed promptly with supplemental oxygen
and prompt descent to lower altitudes.
Neurogenic pulmonary edema may occur within minutes to
hours in patients with acute central nervous system injury, usually in the form
of seizures, intra-cerebral or subarachnoid hemorrhage, or head trauma. The
exact pathophysiology is unknown but may involve an abrupt increase in
pulmonary venoconstriction from sympathetic stimulation with subsequent
elevations in capillary hydrostatic pressures, pulmonary microvascular injury,
or both. With supportive care and management of the underlying neurologic
insult, the edema usually resolves within 48 to 72 hours.
Certain drug ingestions can cause pulmonary edema,
including opiates (heroin and methadone), oral or intravenous-agonists used to
manage preterm labor, and salicylates. Again, the exact mechanisms are not completely understood but may
involve a combination of increased pulmonary capillary pressures and altered
vascular permeability. The pulmonary edema from salicylate overdose can be
exacerbated by standard overdose management with volume resuscitation and
alkalinization with intravenous sodium bicarbonate.
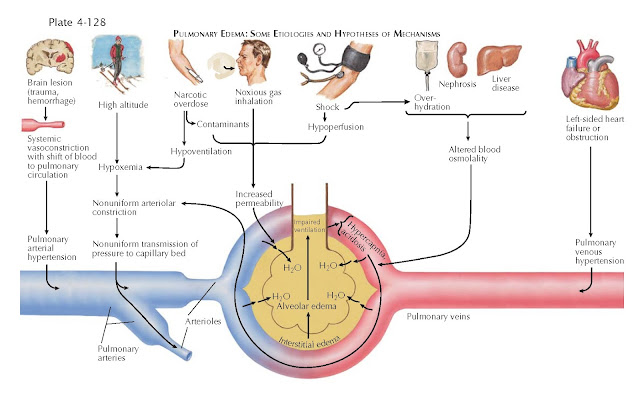
PULMONARY EDEMA: SOME ETIOLOGIES AND HYPOTHESES OF MECHANISMS
EVALUATION OF PATIENTS WITH PULMONARY EDEMA
Patients with pulmonary edema present with the acute
onset of dyspnea, tachypnea, and hypoxemia with radiographic studies showing
bilateral alveolar infiltrates and increased interstitial markings. The history
and clinical context often suggest the cause of the pulmonary edema. Symptoms
consistent with an acute coronary syndrome strongly suggest cardiogenic edema,
although pulmonary edema in the setting of pneumonia, an acute abdomen, or
aspiration points toward ALI. Patients with seizures or intracerebral
hemorrhage may have neurogenic pulmonary edema but could also have had gastric
aspiration during periods of altered consciousness. Older cardiac patients
often are at risk for sepsis syndromes. Thus, although the clinical history is
essential, it is not always definitive as to whether the edema is cardiogenic,
noncardiogenic, or a combination of both.
The physical examination may suggest cardiac disease,
but findings of an S3 gallop or murmurs from valvular disorders may be difficult
to hear in the noisy emergency department or intensive care unit environment.
Lung examination findings of inspiratory crackles are similar in both forms of pulmonary edema.
Peripheral edema is not specific for cardiac disease. Ancillary studies are
obviously important. The electro-cardiogram may show evidence of ischemia.
Laboratory tests assess for evidence of infection, pancreatitis, or drug
ingestions. Plasma levels of brain natriuretic peptide (BNP) are elevated when
cardiac chambers are distended from congestive heart failure or volume
overload. Low BNP levels (100 pg/mL) strongly support a noncardiogenic cause
of pulmonary edema. High levels (500 pg/mL) suggest a cardiac cause, but
intermediate levels are generally not helpful. Direct hemodynamic estimates
of the left atrial pressures are possible with placement of a pulmonary artery
catheter, but this is an invasive procedure with known complications. Use of
these catheters has not been associated with improved patient outcomes.
Imaging of the chest with plain radiographs and
computed tomography scans suggests cardiogenic edema if there are pleural
effusions, an enlarged cardiac silhouette, widened central vascular structures,
septal lines, and peribronchial cuffing. Absence of these features and patchy,
peripheral infiltrates suggest noncardiogenic edema. Bedside transthoracic
cardiac echocardiography can be a quick and noninvasive way to evaluate for
impaired systolic function or valvular disease, but it is less sensitive for
diastolic dysfunction.
The systematic approach to pulmonary edema uses
history, physical exam, laboratory evaluation, and imaging. Echocardiography
and, if needed, invasive hemodynamic monitoring are used in patients in whom the cause of the edema is still not
certain.




