HERPES SIMPLEX VIRUS
Herpes simplex virus type 1 (HSV1) and type 2 (HSV2) are the two viruses that are responsible for the production of both mucocutaneous and systemic disease. Mucocutaneous disease is overwhelmingly more common than systemic disease such as HSV encephalitis. HSV infections are ubiquitous in humans, and almost all adults develop antibodies against one of these viruses. Most infections are subclinical or so mild that they are never recognized by the patient. HSV infections are predominantly oral or genital. The virus becomes latent in local nerves and can be reactivated to produce future outbreaks. Currently, there are eight known herpesviruses that infect humans, including HSV1 and HSV2. HSV infections can cause severe, life-threatening central nervous system (CNS) disease in immunocompromised patients and in neonates. Many unique cutaneous forms of HSV have been described with their own clinical characteristics.
Clinical
Findings: HSV can be spread from infected to uninfected individuals by close
contact (e.g., kissing, sexual contact). The virus is shed from the infected
host both when active lesions are present and when no clinical evidence of
disease can be seen. It is believed that sub- clinical shedding of the virus is
responsible for a great deal of transmission. HSV can cause oral labial disease
(gingivostomatitis or herpes labialis) or genital disease, the main
mucocutaneous forms of the disease. Most cases of oral labial disease are
caused by HSV1, and genital disease is caused predominantly by HSV2. This is
not always the case, and one can no longer assume the viral type from the
clinical location of disease. HSV infections in other areas are becoming more
common, and recurrent bouts of disease on the buttocks is one of the most
frequently seen presentations.
 |
| LESIONS OF HERPES SIMPLEX |
The initial
HSV infection can be subclinical, mild, or severe. Subsequent reactivation of
the virus typically never approaches the severity seen in the initial primary
infection. The exception occurs with immunosuppressed patients, in whom a widespread
or chronic localized version of the infection may occur. Primary infection
manifests with severe, painful mucocutaneous blistering and erosions. Primary
oral labial herpes can lead to weight loss, fever, gingivitis, and pain. This
is most commonly seen in children and is associated with tender cervical
adenopathy. The infection spontaneously resolves within 2 to 3 weeks. If
treated, the disease may be slightly decreased in length and severity, but this
is highly dependent on the timing of diagnosis and initiation of therapy.
Herpes
labialis is
the term given to recurrent episodes of oral labial herpes. The episodes are
milder than the primary infection and often start with a prodrome. Most
patients complain of a tingling or painful sensation hours to a day before the
appearance of herpes labialis. Patients can use this knowledge to their
advantage and begin antiviral therapy at the first indication of recurrence to
decrease the severity of the episode or abort it all together. Herpes labialis,
also known as a cold sore, appears as a vesicle or bulla that quickly breaks
down and forms an erosion and crusted papule or plaque. The lesions last for a
few days to 1
week and can cause significant
psychological issues.
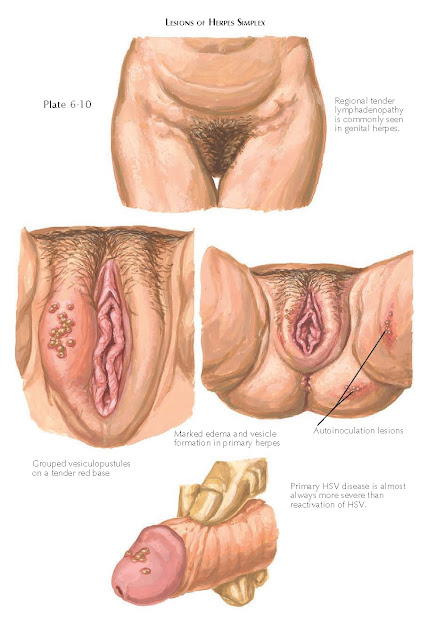
Herpes
infection of the genital region is spread by sexual contact and is one of the
most common of all sexually transmitted diseases. Initial episodes of genital
herpes infection manifest with fever, adenopathy, and painful ulcerations and
blistering of the affected region. The primary episode is always more severe
than subsequent reactivations of the virus. The ulcerations are grouped vesciulopustules
on an erythematous base. They are extremely tender and easily rupture to form
shallow ulcerations that appear “punched out” with an overlying serous crust.
The cervix is often involved, and scarring can occur. Genital herpes infection
almost universall causes dysuria and inguinal adenopathy that is tender.
Recurrent
episodes of genital herpes produce a milder version of the primary infection.
The systemic constitutional symptoms are often absent, but the grouped vesicles
and ulcers can cause excruciating pain and social stigma. The frequency and
severity of recur- rent episodes in an individual patient are variable and
impossible to predict. A generalization can be made that those who have more
severe primary infections tend to have more relentless recurrences.
Herpetic
whitlow is
the name given to a specific form of infection that is most commonly seen in
medical laboratory workers and health care providers. It occurs from accidental
inoculation of the herpesvirus into the skin. The finger is the area most
commonly involved, because of accidental needle sticks. A painful primary viral
infection may occur at the site of inoculation.
Eczema
herpeticum, Kaposi’s varicelliform eruption, is often encountered in a young
child with severe atopic dermatitis who is exposed to the herpesvirus. Because
of the widespread skin disease, the virus is able to infect a large surface
area of the body. This results in extensive skin involvement with multiple
vesicles and punched-out ulcerations.
The
transmission of HSV from mother to child during the birthing process is of
significant concern, and mothers with active HSV disease at the time of
delivery most likely should undergo cesarean section to help decrease the risk
of transmission. Neonatal HSV infection is a life-threatening disease. The
neonate may have widespread multiorgan disease, with CNS involvement being the
major cause of morbidity and mortality. Temporal lobe involvement can lead to
seizures, encephalitis, and death. The skin is always infected, and this is a
clue for the clinician to search for other organ system involvement, especially
involvement of the CNS and the eye. Ocular infection can lead to severe corneal
scarring and blindness.
 |
| LESIONS OF HERPES SIMPLEX |
HSV
encephalitis is a life-threatening disease that causes a necrotizing
encephalitis. Patients complain of an acute onset of fever and headache, with
rapidly evolving seizures and focal neurological deficits. Without treatment,
coma and death occur in three quarters of affected patients. The temporal lobes
and insula are almost always affected. Prompt recognition and therapy have
decreased the mortality rate to 1 in 4. A Tzanck preparation is a long-used
bedside procedure that takes only a few minutes to perform and is positive in
cases of HSV1, HSV2, or varicella-zoster virus (VZV) infection. The procedure
does not differentiate among the three viruses. However, HSV infection can be
distinguished from varicella clinically. The procedure is done by unroofing a
vesicle and scraping its base with a no. 15 blade scalpel. The scrapings are
placed on a glass slide and allowed to air dry for 1 to 2 minutes. A blue stain
such as Giemsa or toluidine blue is applied for 60 seconds and then gently
rinsed off. The slide is dried, mineral oil is applied, and the preparation is
covered with a microscope cover slip. It is then ready to be viewed.
Multinucleated giant cells are readily seen throughout the sample, confirming
the viral etiology of the
blister.
Rapid
immunostaining is available and can be used with high sensitivity and
specificity to diagnose and differentiate the various herpesvirus types. This
form of direct fluorescent antibody (DFA) testing is similar to the Tzanck
preparation. As in the Tzanck preparation, scrapings of the blister base are
placed on a glass microscope slide. The slide is stained with antibodies
corresponding to the various herpesviruses. The sample is viewed under
fluorescent microscopy, and a positive sample fluoresces with one of the specific viral stains. This test takes
1 to 2 hours to perform.
Viral tissue
cultures can also be performed to differentiate the HSV types, but the results
can take days to 1 week to obtain. This is the most sensitive and specific test
for the infection.
Histology:
Examination
of a biopsy specimen of a blister shows ballooning degeneration of the
epidermal keratinocytes. This degeneration forms the blister cavity. There is a mixed
inflammatory infiltrate around the superficial and deep dermal vascular plexus.
Multi- nucleated giant cells are found at the base of the blister pocket. The
skin biopsy findings are unable to differentiate HSV1 from HSV2 or from VZV
infection.
Pathogenesis:
HSV1
and HSV2 are double-stranded DNA viruses encased within a lipid envelope. Along
with VZV, they are classified in the subfamily Alphaherpesvirinae. The five
other human herpesviruses are classified slightly differently. The virus
attaches to the host cells via specialized glycoproteins expressed on its lipid
envelope. The lipid envelope then fuses with the host cell, allowing the virus
to gain entry into the cytoplasm. Many glycoproteins are responsible for this
attachment and fixation and the entrance into the host cell. The HSV capsid,
which is an icosahedron-shaped structure, migrates from the cytoplasm to the
nucleus of the cell. The viral capsid attaches to the nuclear membrane through
the interaction of various membrane proteins and is capable of transferring its
DNA into the cell nucleus.
Once the HSV
DNA has gained entrance into the nucleus, it can become latent and quiescent or
can actively replicate new virus particles. When they are actively replicating,
the HSV particles often have a cytotoxic effect on the affected cell after
viral replication has occurred; this ensures the production of viral progeny
and their release from the host cell. HSV is capable of hijacking the host
cell’s replication protein apparatus. HSV uses the host cell DNA polymerase to
replicate its DNA and uses the cellular machinery to produce proteins required
for viral replication. The virus carries various DNA genes that can be
expressed early during the course of infection or later when the virus is ready
to produce progeny. The early gene products are important for replication and
regulation of the viral DNA genes. The late gene products encode the viral
capsid. Once the viral elements have been produced in sufficient quantity and
in the proper ratio, the viral particles spontaneously converge to produce a
capsid, which encapsulates the viral DNA. This occurs within the host cell
nucleus. The virus then passes through the nuclear membrane and the cytoplasmic
membrane, acquiring its lipid bilayer. At this point, the virus is free to infect
another host.
Alternatively,
after it enters the cell’s nucleus, the virus may become latent. This is
particularly the case in neural tissue. The viral DNA inserts itself into the
host DNA, where it lies dormant and hidden from expression until reactivation
occurs at some later time. It accomplishes this by specialized folding of the
DNA and histone complex so as not to allow for viral gene expression. When the
virus is reactivated and ready to produce viral particles, this mechanism of
latency is somehow deactivated, allowing for viral reproduction.
 |
| HERPES SIMPLEX VIRUS ENCEPHALITIS |
Treatment:
Therapy
and its efficacy are highly dependent on the timing of administration.
Antiviral medications work by inhibiting viral synthesis, and they work best
when used early in the course of disease. Primary infections should all be
treated with one of the antiviral agents in the acyclovir family. These closely related medications
include acyclovir, famciclovir, valacyclovir, and topical penciclovir.
Recurrent episodes of the disease can be treated at the time of outbreak or
with a chronic daily suppressive regimen. Widespread eczema herpeticum, CNS
infection, or infection in an immunosuppressed patient is probably best treated
with intravenous antiviral medication. The acyclovir family of medications are
converted to their active form by viral-specific thymidine kinase. After
conversion, this metabolite
is a potent inhibitor of viral DNA polymerization. These medications are highly
specific for the viral enzymes and have an excellent side effect profile.
Acyclovir-resistant HSV has become well recognized and is best treated with
foscarnet. Foscarnet does not require modulation by thymidine kinase to become
an active inhibitor of HSV replication, thereby bypassing the HSV resistance
mechanism. No medication to date has shown activity
against latent viral infection.




