Transplantation For Diabetes Mellitus
Diabetes mellitus
Diabetes mellitus is characterised by high blood sugars
due to insufficient insulin or insensitivity to the actions of insulin.
Type 1 diabetes is due to an
autoimmune destruction of the insulin-producing beta cells. Patients typically
present in child-hood or adolescence with ketoacidosis and are
insulin-dependent from the outset. Autoantibodies to islet cell antigens are
frequently detectable.
Type 2 diabetes is the result of
insulin resistance, and typically occurs in older and more obese patients. They
are usually non-ketotic at presentation and do not immediately require insulin.
Initially the beta cells attempt to compensate for the insulin resistance by
increasing production; however, with time, the beta cells burn out.
Other forms of diabetes: Gestational diabetes (GDM) – occurs in pregnancy, has similar
features to type 2 diabetes and often resolves after delivery. Many patients
with GDM will go on to develop type 2 diabetes later in life.
Maturity onset diabetes of the young (MODY) – caused by single gene mutations (e.g. HNF-1α gene) that result in
abnormal beta cell function, insulin processing or insulin action.
Pancreatic pathology – pancreatitis,
pancreatic cancer, cystic fibrosis, haemochromatosis and pancreatectomy may all
cause diabetes.
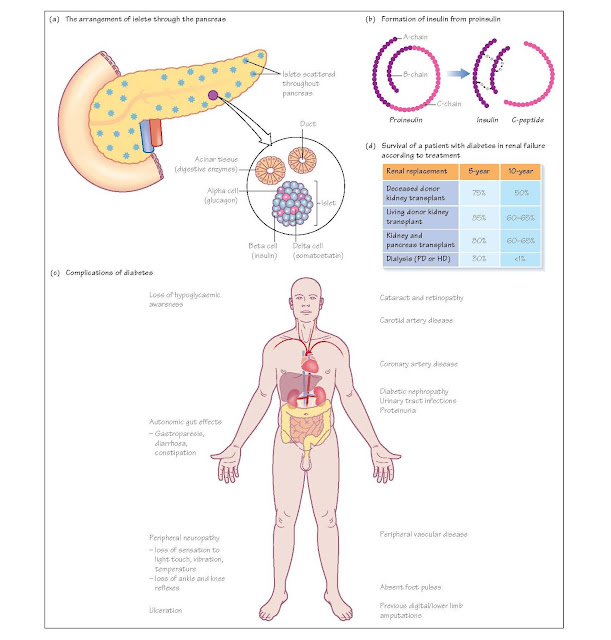
Insulin production
Around 1% of the cells in the pancreas are within the
islets of Langerhans; these are small clusters of hormone-secreting cells that
are scattered throughout the pancreas. One of these hormone-secreting cell
types is the beta cell, which secretes insulin in response to high blood
glucose. The islets also contain other hormonesecreting cells, such as alpha
cells producing glucagon, and delta cells producing somatostatin.
Within the beta cells insulin is produced as a precursor
called proinsulin, a single polypeptide chain which folds such that the two
ends of the chain become bound by two pairs of disulphide bonds. This
polypeptide is then cleaved into three fragments, the A, B and C peptides. A
and B form the insulin molecule, and the C peptide is released. Measurement of
C peptide in the serum can be used to determine whether a potential recipient
makes their own insulin (i.e. not type 1), since artificial insulin does not
contain this peptide.
High concentrations of glucose entering the beta cells
trigger release of insulin. This insulin is secreted directly into the portal
circulation to have its initial effect on the liver, where it is required to
permit entry of glucose into the cells.
The complications of diabetes
The main complication of diabetes is the development of
accelerated vascular disease. This is particularly marked in patients with poor
glucose control and those who smoke. Vascular complica- tions are categorised
according to the size of vessels involved:
Macrovascular complications
1 Coronary artery disease: angina and/or myocardial infarction.
2 Peripheral vascular disease (PVD) characterised by claudication, rest pain, ulceration and gangrene.
3 Cerebrovascular disease, manifesting with transient ischaemic attacks (TIA), amaurosis fugax or
cerebrovascular accident.
Microvascular complications
Retinopathy Microvascular
disease affecting the retinal vessels is classified according to severity and
whether the macula is involved.
- Background – microaneurysms (dots) and microhaemorrhages (blots), hard exudates.
- Pre-proliferative – cotton wool spots (soft exudates indicative of retinal infarcts), more extensive microhaemorrhage.
- Proliferative – new vessel formation.
- Maculopathy – changes described in background or pre-proliferative retinopathy affecting the macula.
If there is significant haemorrhage then retinal
detachment may occur. Diabetes is also associated with cataract formation.
Neuropathy A number of types
of diabetic neuropathy occur.
Peripheral sensory neuropathy – typically in a ‘glove and stocking’ distribution. Vibration sensation is
lost early. In advanced disease, sensation in the feet may be completely
absent, resulting in unnoticed trauma and subsequent ulceration. In the
presence of PVD, the blood supply is impaired, leading to poor healing,
sometimes necessitating amputation.
Autonomic neuropathy – symptoms vary
and include gustatory sweating, gastroparesis (vomiting and nausea), bladder
dysfunction, erectile dysfunction and postural hypotension (due to loss of
regulation of vascular tone). Of most significance is the loss of awareness of
hypoglycaemia. Hypoglycaemia is normally accompanied by tremor, sweating and
palpitations due to the release of adrenaline (epinephrine) in response to low
brain glucose (neuroglycopaenia). This has the additional role of stimulating
glycogenolysis and gluconeogenesis in the liver. This compensatory adrenaline
release is lost in patients with hypoglycaemic unawareness. The net result is
that blood sugar may fall dangerously low, causing significant brain damage or
death.
Painful neuropathy – damage to sensory
nerves may lead to a burning pain or sensitivity to touch.
Mononeuritis multiplex – may affect any
peripheral nerve.
Diabetic amyotrophy – painful wasting
and weakness of quadriceps.
Nephropathy Patients with
type 1 diabetes frequently develop renal involvement. At least 25% of diabetics
diagnosed before the age of 25 years will go onto to develop end-stage renal
failure.
Diabetic nephropathy is characterised by albuminuria,
which may progress to heavy proteinuria with decline in glomerular filtration
rate (GFR). Histologically, there is basement membrane thickening and
glomerulosclerosis, which may be diffuse or nodular (Kimmelstiel–Wilson
lesions). Diabetic patients are also more susceptible to urinary tract
infections, which may contribute to chronic renal damage.
In the UK, diabetes is the most common cause of ESRF
requiring renal replacement therapy. Diabetics on dialysis have a very poor
outlook, with a 30% 5-year survival.
Indications
Both pancreas and islet transplantation are for the
treatment of diabetes mellitus. Since both require standard immunosuppression,
the benefits of the procedure have to outweigh the risks, and the side effects
and complications of immunosuppression. There- fore it is generally agreed that
the patient should have a life-threatening complication of diabetes, such as
hypoglycaemic unawareness, or that they require immunosuppression for another reason,
such as a kidney transplant.