FOLLOWING
PROTEIN MOVEMENTS AND INTERACTIONS
A variety of methods have been developed to follow the movement and interactions of GFP-labeled proteins within living cells. One widely used method for studying the movements of GFP-labeled proteins is fluorescence recovery after photobleaching (FRAP) (Figure 1.31). In this technique, a region of interest in a cell expressing a GFP-labeled protein is bleached by exposure to high-intensity light. Fluorescence recovers over time due to the movement of unbleached GFP-labeled molecules into the bleached region, allowing the rate at which the protein moves within the cell to be determined.
 |
Figure
1.31 Fluorescence recovery after photobleaching (FRAP) A region of a
cell expressing a GFP-labeled protein is bleached with a laser. Fluorescence
recovers over
time as unbleached GFP-labeled molecules diffuse into the bleached region. The
rate of recovery of fluorescence therefore provides a measurement of the rate
of protein
movement within the cell.
The interactions of two proteins with one another within a cell can be analyzed by a technique called fluorescence resonance energy transfer (FRET) (Figure 1.32). In FRET experiments, the two proteins of interest are coupled to different fluorescent dyes, such as two variants of GFP. The GFP variants are chosen to absorb and emit distinct wavelengths of light, such that the light emitted by one GFP variant excites the second. Interaction between the two proteins can then be detected by illuminating the cell with a wavelength of light that excites the first GFP variant and analyzing the wavelength of emitted light. If the proteins coupled to these GFP variants interact within the cell, the fluorescent molecules will be brought close together and the light emitted by the first GFP variant will excite the second, resulting in emission of light at the wavelength characteristic of the second variant of GFP.
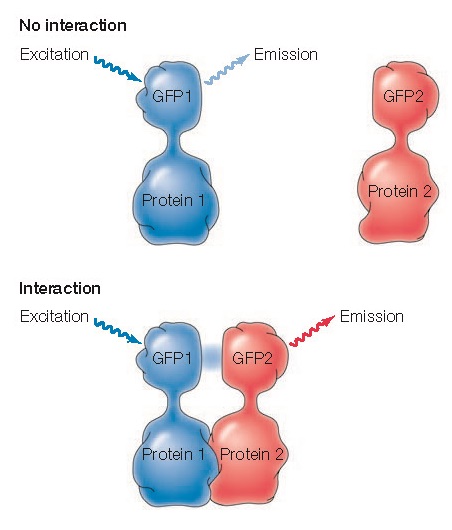 |
Figure
1.32 Fluorescence resonance energy transfer (FRET) Two
proteins are fused to different variants of GFP (GFP1 and GFP2) with
distinct wavelengths for excitation and emission, chosen such that the
light emitted by GFP1 excites GFP2. Cells are then illuminated with a wavelength
of light that excites GFP1. If the proteins do not interact, light emitted
by GFP1 will be detected. However, if the proteins do interact, GFP1
will excite GFP2, and light emitted by GFP2 will be detected.




