PULMONARY EMBOLISM AND VENOUS THROMBOEMBOLISM
Pulmonary embolism (PE) and deep venous thrombosis (DVT) are generally considered to be two clinical presentations of venous thromboembolism (VTE). In most cases, PE is a result of embolization of clot from DVT. The diagnosis and management of patients with PE have been addressed in a number of summary articles and guidelines, including guidelines prepared by a Task Force of the European Society of Cardiology.
 |
| PREDISPOSING FACTORS FOR PULMONARY EMBOLISM |
RISK FACTORS FOR PULMONARY EMBOLISM
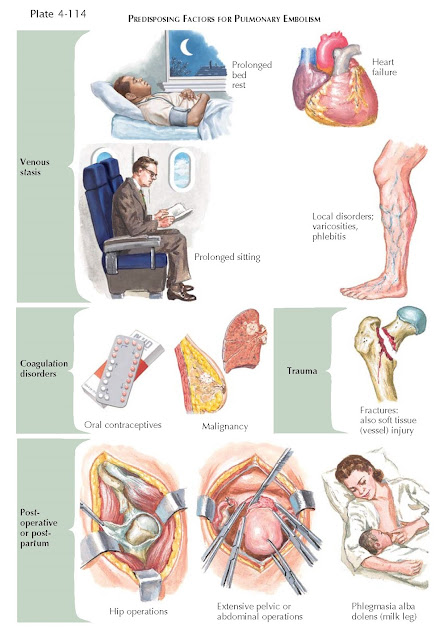
PE can occur without identifiable predisposing factors,
but one or more factors are usually identified, such as age, history of previous
DVT, cancer, neurologic disease with paresis, medical disorders associated with
prolonged bed rest, thrombophilia, hormone replacement therapy, and oral
contraceptive therapy (see Plate 4-114). There may also be associations with
obesity, smoking, and systemic hypertension or the metabolic syndrome. Surgery,
particularly orthopedic surgery, is associated with an increased risk of PE.
PATHOPHYSIOLOGY
The source of clots is generally the deep veins of the
legs and pelvis (i.e., a femoral, popliteal, or iliac vein) (see Plate 4-115).
Most often, clots in a thigh vein originate as an extension of a clot in a deep
calf vein. Superficial thrombophlebitis in the legs or thighs rarely gives rise
to emboli but may signal a DVT. The loose propagating thrombus in the deep
veins constitutes the hazard of pulmonary embolization. When broken loose, the
clot is carried to the lungs through the venous stream and right side of the
heart.
Superficial thrombophlebitis, which may be associated
with DVT, occurs in fewer than one-third of patients with PE. Signs of DVT in
the calf or thigh are difficult to detect until the venous circulation is
extensively compromised (see Plates 4-116 and 4-117). When careful examination
fails to implicate veins of the extremities, it is usual to suspect thrombosis of less accessible deep veins, particularly
the pelvic veins in women who have had complicated obstetric manipulations,
pelvic inflammatory disease, or septic abortion associated with suppurative
pelvic thrombophlebitis.
Local or systemic disorders that predispose to venous
thrombosis in the legs are also potential precursors of pulmonary emboli (see
Plate 4-114). Paramount among these is venous stasis. Even in a normal person,
a pro-longed ride with flexed knees in an automobile or air plane may lead to venous stasis and
thrombosis in the legs.
CLINICAL MANIFESTATIONS OF LEG VEIN THROMBOSIS
Clinical manifestations of thromboses in the leg veins
remain an important part of disease recognition and prompt diagnosis (see Plate 4-116).
 |
| SOURCES OF PULMONARY EMBOLI |
History
Thrombophlebitis is usually brought to the patient’s
attention by pain in the muscles of the affected leg. The pain may be diffuse
or localized, and the patient usually does not confuse it with joint pain.
Patients may notice that the pain is far worse on dependency and, conversely,
completely relieved by elevation. There is often swelling of the affected leg
and foot; the extremity may be warm locally, and the patient may be febrile.
Certain circumstances are likely to be associated with
DVT, and the physician should review these points with the patient. An initial
event may be dependency of the leg for several hours. Obesity; chronic illness,
particularly carcinoma and most particularly carcinoma of the pancreas; and use
of oral contraceptives enhance the possibility of this complication.
Physical Examination
The patient should first be examined in the standing
position. The presence of varicose veins should be noted because they increase
the patient’s susceptibility to thrombophlebitis. Enhancement of the pain by
dependency may provide a useful diagnostic clue. The patient is then examined
in the recumbent position. A valuable method of detecting unilateral
thrombophlebitis is to evaluate the tissue consistency of the affected leg
compared with that of the unaffected leg. The examination should be preceded by
palpation of the calves for tenderness with the patient’s leg slightly flexed.
Generalized tenderness of the calf or thigh may be found. In addition, there
may be tenderness along the major veins of the calf or thigh and superficial
point tenderness of small segments of veins involved with thrombophlebitis. The
finding of superficial phlebitis is most important in that the potential for
complicating thromboembolism is much less when a segment of vein is tender and
a thrombus can be felt but there is little or no tenderness elsewhere. The area
of thrombosis may appear red because of inflammation spreading to the skin.
Homans sign is difficult to
evaluate. The problem is that the tenderness may be bilateral. Elderly people,
particularly, experience some pain in their calves with dorsiflexion of their
feet.
One of the main techniques for diagnosing and
following a patient is that of comparative circumferential measurements of the
legs at several levels. The aim is to look for minor amounts of edema that are
not readily apparent. A difference of as little as 0.5 cm may be significant. Normally, the patient’s
dominant leg may be slightly larger than the other leg. This normal increase
may be as much as 2 cm at the calf and more in the thigh.
Finally, a serious complication (phlegmasia cerulea
dolens) that may arise is the absence of arterial circulation in the affected
leg. This represents a medical emergency in that the reflex reduction of
arterial circulation, as a relatively infrequent complication of thrombophlebitis, may lead to gangrene of the tissues
of the foot. The diagnosis is made by observation of the deepening blue color
of the extremity as well as the lack of arterial pulses and coldness of the
distal part of the extremity in contrast to the usual warm state in
uncomplicated thrombophlebitis.
DIAGNOSIS OF DEEP VENOUS THROMBOSIS (see Plate 4-117)
Ultrasonography
In 90% of cases, PE originates from lower extremity
DVT. Lower limb compression venous ultrasonography (CUS) has largely replaced
venography for diagnosing DVT. For proximal DVT, CUS has a sensitivity of more
than 90% and a specificity of approximately 95%.
Computed Tomography Venography Computed tomography (CT) venography has been recently
advocated as a simple way to diagnose DVT in patients with suspected PE because it can be combined with chest CT angiography in a
single procedure using only one intravenous injection of contrast dye. However,
it appears as though CT venography increases the overall detection rate only
marginally in patients with suspected PE and adds a significant amount of
irradiation.
CLINICAL MANIFESTATIONS OF PULMONARY EMBOLISM
The clinical manifestations of pulmonary embolization
are generally subtle, unexplained tachypnea and dyspnea; anxiety; vague
substernal pressure; and occasionally syncope. In a patient predisposed to PE
by bed rest, surgery, or local thrombophlebitis, these symptoms constitute
strong evidence for a pulmonary embolus even though the physical examination is
unrewarding, the electrocardiogram (ECG) indeterminate, and the chest
radiograph normal.
The most common type of PE is one that does not result
in infarction (see Plate 4-118). This is because of the protective effect of
the dual pulmonary circulation that protects the lung from infarction except in
cases of massive embolus or in patients with
concomitant left-sided heart failure.
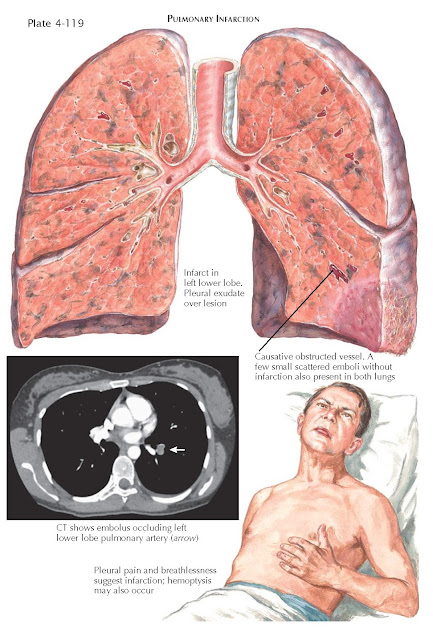
PE resulting in infarction occurs after less than 10%
of pulmonary emboli. The evidence for pulmonary infarction is acute onset of
pleural pain, hemoptysis, breathlessness, pleural effusion, or pleural friction
rub (see Plate 4-119).
A massive embolus that either lodges in the main
pulmonary artery or overrides both branches to the point of compromising the bulk of
the pulmonary blood flow is a disaster that elicits circulatory collapse and
acute cor pulmonale (see Plate 4-120). This form of pulmonary embolization is a
dire emergency, but it is difficult to distinguish from an acute myocardial
infarction. The chances of detecting it depend on the physician’s suspicion
that the patient is predisposed to pulmonary embolization. After clinical
suspicion has been raised, support for the diagnosis is provided by the classic S1-Q3 pattern on the
ECG. Almost as convincing is a fresh “P pulmonale” pattern, a new right- axis
shift, or a new pattern of incomplete right bundle-branch block.
The effect of one or more massive emboli is a
reduction in the cross-sectional area of the pulmonary vascular tree and an
increase in pulmonary vascular resistance to blood flow. If most of the
pulmonary vascular tree is blocked, marked pulmonary hypertension occurs
followed by dilatation and even failure of the right ventricle. In patients
with previously normal lungs, the severity of these changes correlates closely
on a lung scan with the extent of perfusion defects. Whether the total
hemodynamic effect is attributable to the restricted vascular bed or to
associated reflex or humoral vasoconstrictor mechanisms is unclear. A decrease
in cardiac output and a decrease in systemic blood pressure accompany the right
ventricular enlargement. Preexisting cardiac or lung disease aggravates these
changes and may precipitate intractable heart failure.
When PE is extensive enough to produce acute
right-sided heart failure, it often results in syncope and cardiopulmonary
arrest. Profound apprehension, central chest pain, and cardiac dysrhythmias
(especially atrial flutter) may also occur, and in many patients, death follows
within a few hours of the embolic episode. The physical findings of acute cor
pulmonale include tachy- cardia, an elevated jugular venous pressure with
prominent A wave, shock, and cyanosis. Wide splitting of the second heart sound
may be present and is often fixed. It disappears with the resolution of the embolus
and relief of right ventricular failure. Occasionally, a right ventricular
gallop can be heard along with a systolic ejection murmur in the pulmonary
area. There may be a palpable lift over the right ventricle and a loud
pulmonary closure sound.
DIAGNOSIS OF PULMONARY EMBOLISM
Chest Radiography
The radiographic appearance depends on the size and
number of emboli, whether they have produced pulmonary infarction, and whether
the infarcted area reaches the
pleural surface to cause pleuritis and pleural effusion. A massive embolus located at the origin of a
major pulmonary artery causes hypoperfusion of the ipsilateral lung manifested
by a decrease in vascular markings. An increase in size of a major hilar vessel
or an abrupt cutoff, the “knuckle sign,” is strong supportive evidence when
present. If not distinctly oligemic, areas of the lung often show unduly small
vessels. Sometimes the only indication of a large embolus is an unusually high
diaphragm on the affected side or the
presence of a pulmonary infiltrate, a consequence of infarction, hemorrhage, or
atelectasis. An ipsilateral pleural effusion may also be the only sign of an
otherwise unsuspected pulmonary infarction. All of this radiographic evidence
takes on a great significance if the individual is predisposed to peripheral or
pelvic venous thrombosis and has been identified as a serious c ndidate for PE.
Often nothing abnormal can be seen.
Arterial Blood Gases
A mainstay in the diagnosis of massive PE is a
decrease in arterial oxygen tension, generally in association with reduced
arterial carbon dioxide tension. Whereas the arterial hypoxemia is a
consequence of ventilation/perfusion (V/Q) abnormalities, the hypocapnia is
caused by hyperventilation that is presumed to be reflexly induced by the emboli
via the J receptors. Hypoventilated areas probably result from interference
with surfactant and resulting atelectasis in small areas of lung.
D-Dimer
Plasma D-dimer levels, a measurement of a degradation
product of cross-linked fibrin, are elevated in plasma in the presence of an
acute clot caused by simultaneous activation of coagulation and fibrinolysis. A
normal D-dimer level makes acute PE or DVT unlikely. The negative predictive
value of D-dimer is high. Unfortunately, because of the poor specificity of
fibrin for VTE related to the fact that fibrin is produced in a wide variety of
conditions, the positive predictive value of D-dimer is low. D-dimer is not
useful for confirming PE. When measured by quantitative enzyme-linked
immunosorbent assay, D-dimer has a sensitivity of more than 95% and a
specificity of about 40%. D-dimer levels can therefore be used to exclude PE in
patients with a low or moderate probability of PE.
Ventilation/Perfusion Lung Scan
A lung scan, using a radioisotope as a marker, is often
performed to evaluate patients with a suspected diagnosis of PE.
Macroaggregated albumin, labeled with iodine 131 or technetium 99, is commonly
used for this purpose. The tracer substance is injected intravenously. The
radioactive particles, which are on the order of 50 to 100 m in diameter, are
trapped in the microcirculation of the lung. The pattern of distribution of
these radioactive particles, detected by an external counter, defines the
pattern of pulmonary blood flow. It is helpful to have V/Q scans performed at
the same sitting so that areas of inadequate blood flow may be related to
ventilation abnormalities. Most specific in reaching a diagnosis is the finding
of multiple perfusion defects in normally ventilated lungs.
Lung scans are practical, simple, and safe. They can
be repeated as necessary to trace the resolution of defects and to detect fresh
emboli. Results are frequently
characterized according to criteria established in the North American PIOPED
(Prospective Investigation of Pulmonary Embolism Diagnosis) trial into four
categories: normal or near-normal, low, intermediate (nondiagnostic), and high
probability of PE. A normal perfusion scan virtually excludes PE. A
high-probability V/Q scan suggests the diagnosis of PE with a high degree of probability,
but further tests may be
considered in selected patients with a low clinical suspicion of PE. In other
combinations of V/Q scan results and clinical probability, further testing
should be performed.
Computed Tomography
Recent studies have supported the value of CT
angiography in the diagnosis of acute PE. Multidetector CT (MDCT) with high spatial and temporal resolution and
quality of arterial opacification allows adequate visualization of the pulmonary
arteries to at least the segmental level. MDCT may be adequate for excluding PE
in patients without a high clinical probability (suspicion) of PE. Whether
patients with negative CT results and a high clinical probability should be
further investigated (with compressive ultrasonography of the lower extremities
or V/Q scanning or pulmonary angiography) is controversial. A MDCT showing PE
at the segmental or more proximal level is considered adequate proof of PE in
patients without a low clinical probability.
Pulmonary Angiography
The pulmonary angiographic diagnostic criteria for
acute PE were defined many years ago and include direct evidence of a thrombus,
either a filling defect or amputation of a pulmonary arterial branch. Pulmonary
angiography is, however, invasive and carries some risk. However, when
performed by experienced operators, it can be an important confirmatory test.
Echocardiography
The echocardiographic finding of right ventricular
dilatation may be useful in risk stratifying patients with suspected high-risk
PE presenting with shock or hypotension. A meta-analysis found a more than
twofold increased risk of PE-related mortality in patients with
echocardiographic signs of right ventricular dysfunction.
Diagnostic Strategies and Algorithms
Pulmonary angiography, the definitive test, is invasive,
costly, and carries some risk. Therefore, noninvasive diagnostic approaches are
warranted, and various combinations of clinical evaluation and the
above-described tests (including D-dimer measurement, lower extremity
compressive ultrasonography, V/Q scanning, and CT scanning) have been evaluated
to decrease the need for pulmonary angiography. It is important to note that
the diagnostic approach to PE may vary according to the local availability of
tests. The most appropriate diagnostic strategy should also be determined by
the clinical assessment of risk and severity. Various guidelines have been
developed that describe diagnostic strategies and algorithms in detail.
PROPHYLAXIS AND TREATMENT
Prophylaxis
Prophylaxis of VTE is concerned with the prevention of
clot formation in the deep veins of the legs and with the extension of a clot
that can break off and travel to the lungs. Because of the morbidity and
mortality associated with DVT and PE, appropriate prophylaxis is of paramount importance. Specific
guidelines for prophylaxis of VTE have been published by the American College
of Chest Physicians (ACCP).
Anticoagulation After Pulmonary Embolism
Anticoagulant therapy plays a critically important
role in the management of patients with PE. The objectives are to prevent death and recurrent events with an
acceptable risk of bleeding-related complications. Rapid anticoagulation
requires parenteral therapy, such as intravenous unfractionated heparin (UFH),
subcutaneous low-molecular-weight heparin, or subcutaneous fondaparinux.
Because of the high mortality rate in untreated patients, anticoagulation
should be considered in patients with suspected PE while awaiting diagnostic
confirmation. Specific guidelines for anticoagulation after PE have been published
by the ACCP and are updated regularly. The use of intravenous UFH requires
close monitoring of the activated partial thromboplastin time. Treatment with
parenteral anti-coagulants is usually followed by the use of oral vitamin K
antagonists, such as warfarin. Chronic anticoagulation with warfarin requires
ongoing monitoring of the prothrombin time or the International Normalized
Ratio. Protocols to guide anticoagulant dosing and monitoring and follow-up by
a dedicated team of experienced professionals may help to optimize the safety
and efficacy of therapy. Drug interactions can be troublesome during warfarin
therapy, and each new medication must be examined for its effect in enhancing
or diminishing the action of warfarin.
Thrombolysis
Thrombolytic therapy rapidly resolves thromboembolic
obstruction and has beneficial effects on hemodynamic parameters. However, the
benefits of thrombolysis over anticoagulation with heparin appear to be largely
confined to the first few days. Thrombolytic therapy carries a significant risk of
bleeding, especially in patients with predisposing conditions or comorbidities.
Nevertheless, thrombolytic therapy may be used in patients with high-risk PE
presenting with cardiogenic shock or persistent systemic hypotension. Further studies
are needed to more precisely define the role of thrombolytic therapy for PE.
Surgical Pulmonary Embolectomy for Acute Pulmonary
Embolism
Pulmonary embolectomy may be indicated in patients
with high-risk PE in whom thrombolysis is absolutely contraindicated or has failed.
Caval Filters
Inferior vena cava (IVC) filters may be used when there
are contraindications to anticoagulation and a high risk of VTE recurrence (see
Plate 4-121). They are also often placed in patients with chronic
thromboembolic pulmonary hypertension (CTEPH) to provide an additional barrier
of protection against recurrent PE. Some filters in use today are retrievable
and removable and may be suitable for temporary use.
CHRONIC EFFECTS OF PULMONARY EMBOLISM
Chronic Thromboembolic Pulmonary Hypertension
PEs are occasionally dispatched to the lungs for
months to years without clinical evidence of acute embolizations. The patients
may present with evidence of severe pulmonary hypertension and often die in
right ventricular failure. The course of
patients with multiple pulmonary emboli may be so subtle as to mimic that of
patients with idiopathic pulmonary arterial hypertension. CTEPH is a relatively
rare complication of pulmonary thromboembolic disease. It is often
characterized by progressive dyspnea and hypoxemia and ultimately the
development of right-sided heart failure (see Plate 4-121).
In these patients with severe pulmonary hypertension,
dyspnea and tachypnea, fatigue and syncopal episodes, or precordial pain during
exertion are usually found in some combination. On physical examination, an
impulse may be felt over the main pulmonary artery, and there is splitting of
the second heart sound with accentuation of the pulmonary component. An
ejection click and a systolic or diastolic murmur may be present in the
pulmonary valve area. Subsequently, evidence of right ventricular hypertrophy
is found, with a prominent A wave in the jugular venous pulse and a right
ventricular heave and fourth heart sound. As failure develops, a right ventricular
gallop can be heard, and there is evidence of tricuspid valve insufficiency
along with the peripheral consequences of an ineffectively functioning right
ventricle. Sudden death caused by transient arrhythmias may occur.
Chest radiographs usually show an enlarged heart with
right ventricular and right atrial prominence. The main pulmonary artery shadow
is increasingly enlarged as hypertension becomes more severe, and the
peripheral lung fields are oligemic and lack vascular markings. Evidence of right-axis
deviation appears on the ECG, with evidence of right ventricular hypertrophy in
the precordial leads. There is usually indication of right atrial enlargement,
and when changes are severe, inversion of right precordial T waves. Right-sided
heart catheterization and radioisotope lung scans provide definitive evidence of
the disease process.
 |
| MECHANICAL DEFENSES AGAINST AND CHRONIC EFFECTS OF PULMONARY EMBOLISM |
Pulmonary Thromboendarterectomy
Surgical removal of obstructing material related to
chronic thromboembolic disease requires a true endarterectomy rather than an embolectomy. The
operation is performed on cardiopulmonary bypass, with deep hypothermia and
complete circulatory arrest. Selection of appropriate candidates for the
operation is extremely important, and criteria include factors such as surgical
accessibility and the absence of severe comorbidity. PTE carries substantial risk, but in
experienced hands, it may result in dramatic clinical and hemodynamic
improvement. Medical therapy for patients with CTEPH is being explored in
clinical trials.