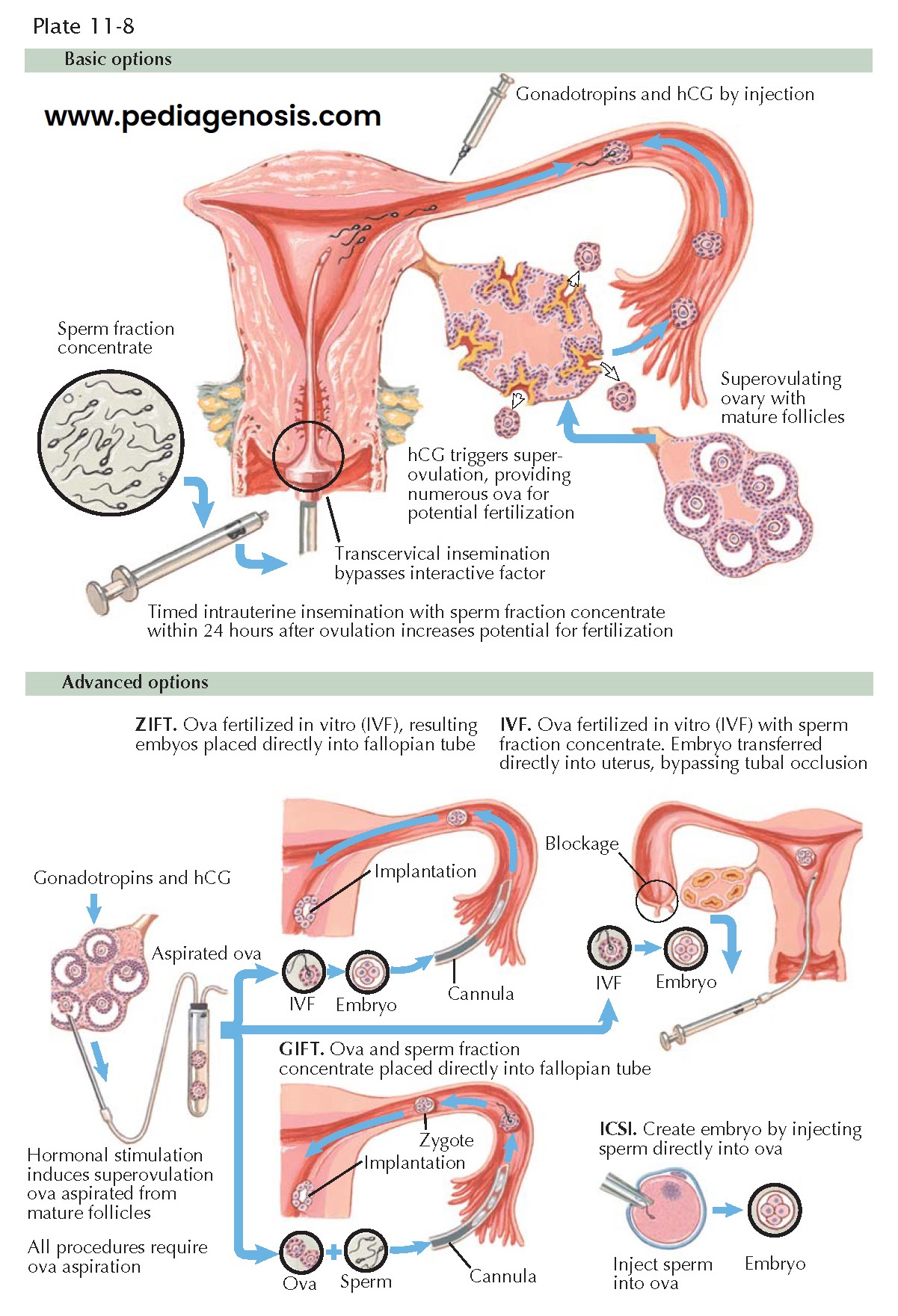
ASSISTED REPRODUCTION
The success of infertility treatment
depends to a great extent on the identified cause. Success is also a function of
the age of the female partner: success declines and the rate of spontaneous pregnancy
loss increases rapidly after age 35, adversely impacting the couple’s ability to
achieve a successful outcome.
A number of techniques are available to accomplish conception. Most are less exotic than their acronyms suggest. Among infertile couples seeking treatment, 85% to 90% can be treated with conventional medical and surgical procedures and do not require advanced assisted reproductive technologies such as in vitro fertilization. Sometimes the solution is as simple as improved timing of intercourse: when couples have intercourse four or more times per week, more than 80% achieve pregnancy in the first 6 months of trying. By contrast, only about 15% of couples conceive when intercourse happens less than once a week. Intercourse should be maintained on an every-other-day cycle from 3 to 4 days before the presumed ovulation until 2 to 3 days after that time. The use of ovulation detection kits (which detect urinary evidence of the luteinizing hormone [LH] surge) can facilitate this process.
Approximately 20% of infertile women have ovulatory disorders. For these women,
ovulation induction or control may be used to enhance the likelihood of
pregnancy. The cause of anovulation will guide the selection of an appropriate treatment
plan; some will need indirect therapies such as weight loss or metformin (polycystic
ovary syndrome), others more direct hormonal manipulations such as afforded by clomiphene
citrate, tamoxifen, aromatase inhibitors, or gonadotropins. All types of assisted
reproductive technologies involving ovarian stimulation are associated with an increased
incidence of multiple gestations (up to 40%): the majority of these pregnancies
are twins (25%), and 5% are higher order gestations.
Tubal factor infertility may be addressed by either surgical repair of the
damage or by bypassing the tubes completely through in vitro fertilization and embryo
transfer (IVF/ET). In vitro fertilization accounts for only about 5% of infertility
services and is associated with a roughly 32% live delivery rate per egg retrieval
(2004). Success rates for surgical repair, including the reversal of previous sterilization
procedures are highly variable.
When male factors such as azoospermia are present, technologies such as intrauterine
insemination (IUI) with donor sperm increase the chance of fertilization. In males
with azoospermia there are two major categories—obstructive or nonobstructive azoospermia.
Males with obstructive azoospermia may often have sperm retrieved from the epididymis.
In males with cystic fibrosis and azoospermia, it is important to test the female
partner to determine if she is a carrier of a cystic fibrosis allele. Males with
nonobstructive azoospermia have varying degrees of primary testicular failure, which
may be secondary to chromosomal abnormalities such as Klinefelter syndrome (47,
XXY). Sperm may be able to be retrieved from the testicle in males with nonobstructive
azoospermia. Sperm retrieved from the testicle or epididymis may result in
successful fertility using intracytoplasmic sperm injection (ICSI), even with as
few as one sperm per oocyte. When either partner is incapable of supplying the
necessary gametes, donor sperm or oocytes may be used to accomplish a pregnancy.
In vitro fertilization and embryo transfer have allowed unprecedented access to gametes and the early developing embryo. Recent genetic developments, including the human genome project, the ability to amplify the DNA from a single cell, and new diagnostic tests, in combination with micromanipulation in IVF (biopsy of a single or two blastomeres), have allowed for preimplantation genetic diagnosis (PGD). PGD allows one to evaluate an embryo in a high-risk couple for the presence of the abnormal genes and selecting unaffected (nor al or heterozygotes) embryos for uterine transfer.