Anatomy of the Basal Ganglia and Related Structures
OVERVIEW OF MOVEMENT DISORDERS
For the past 30 years, movement disorders have encompassed the study of a group of conditions characterized by poverty of movement, the akinetic-rigid syndromes, and those with excessive movements, the hyperkinetic movement disorders (tremor, dystonia, myoclonus, chorea/ballism, tics, and others). This traditional view, in which disorders of basal ganglia resulted in the aforementioned syndromes, has now expanded to include the ataxias and disorders of gait and posture. Advances in surgical techniques and imaging studies have broadened the clinical horizon and catchments of the movement disorders specialist. With the increasing indications for botulinum toxin therapy, spasticity and others disorders are now managed by many movement disorders neurologists.
Abnormal
involuntary movements (AIMs) should be viewed as clinical signs with many causes. For
example, parkinsonism may be the clinical manifestation of a variety of
conditions with different or unclear etiolo gies. Defining
the broad category of the movement dis order in
a given patient precedes the classic approach to neurologic diagnosis: localizing
the lesion and determin ing the etiology
of the condition. A careful history with particular attention to family
background, pregnancy, labor and delivery, early developmental milestones,
trauma, infections, medical and psychiatric comorbidities, and use of illicit
drugs and medications, especially neuroleptics, are particularly important when
first evaluating a patient with abnormal involuntary movements and may suggest
the underlying cause. A detailed general medical examination with emphasis on
eye movements, presence of KayserFleischer
rings (suggesting Wilson
disease), and funduscopic examination looking for retinopathy and optic nerve
abnormalities (papillitis, papilledema, or optic nerve atrophy suggesting
demyelinating diseases, metabolic disorders, or mitochondrial
cytopathies); organomegaly (betraying
metabolic or storage diseases); and skin discolorations or deposits (defining
phakomatosis, xeroderma pigmentosum, vitaminosis, gastrointestinal disease,
malabsorption, calcinosis, or cholesterol deposits, especially at the muscle
tendons) may prove rewarding. Searching for additional clues, with a carefully
performed neurologic examination, will help in the understanding of the
patient’s condition.
Once the
abnormal movements have been classified, and the neurologic accompaniments
documented and placed in context, the cause may become apparent and proper
ancillary testing may be undertaken.
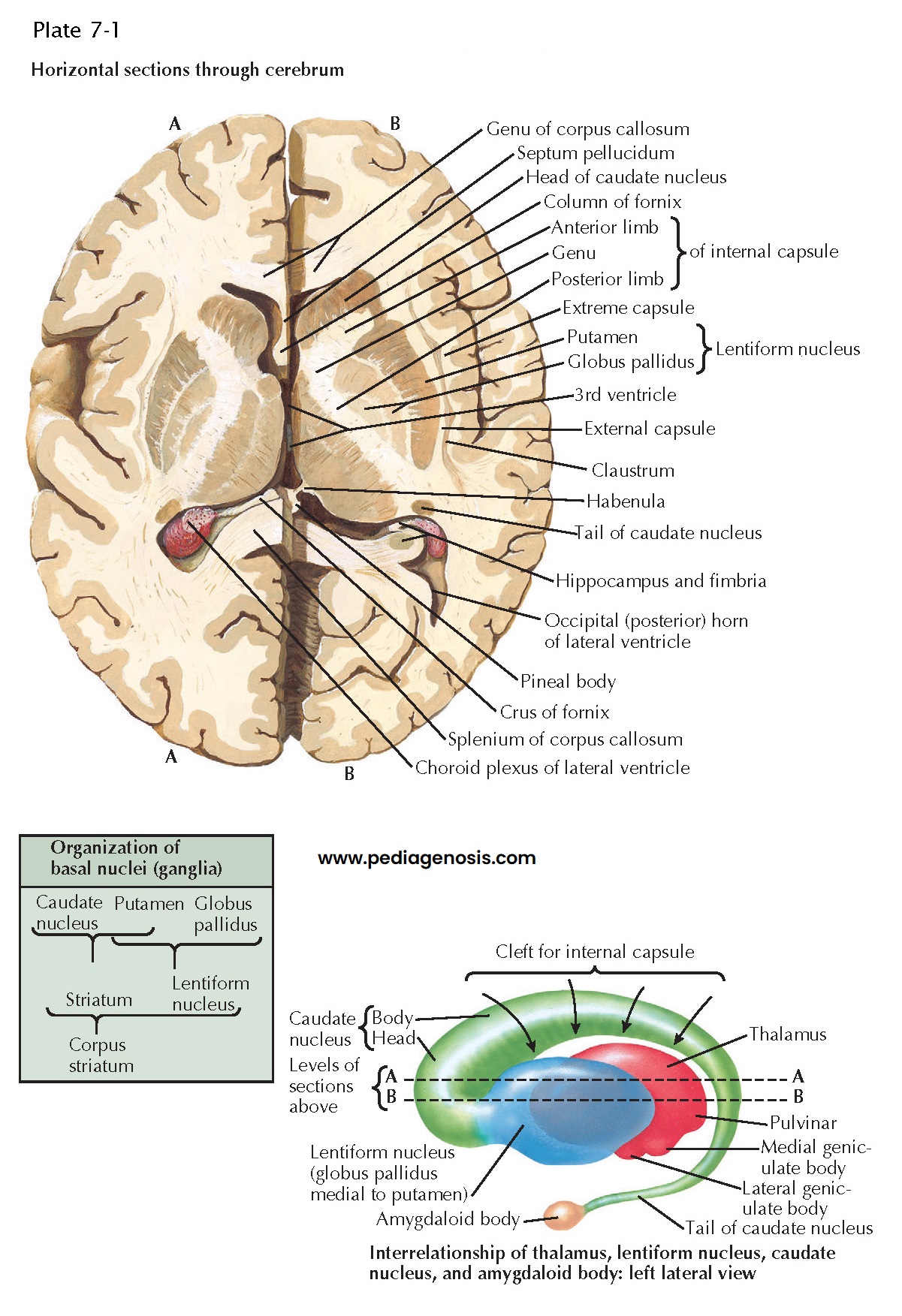
BASAL GANGLIA AND RELATED STRUCTURES
ANATOMY OF
THE BASAL GANGLIA AND RELATED STRUCTURES
Anatomically,
the basal ganglia constitute a complex circuitry that includes neurons of the
caudate nucleus, putamen, subthalamic nucleus (STN) globus pallidus, and
substantia nigra (SN). The output of the basal ganglia is directed at the motor
thalamus (and from there to the frontal cortex) and the pedunculopontine
nucleus (PPN).
Globus
Pallidus. Divided by the internal medullary lamina into an external (GPe) and
internal (GPi) segments, the globus pallidus borders laterally with the
putamen, dorsomedially with the internal capsule and optic tract and ventrally
with the substantia innominata, which, in turn, contains three major functional
anatomic systems: the ventral striatopallidal system, the extended amygdala,
and the nucleus basalis of Meynert. The latter nucleus, with its cholinergic
andγaminobutyric
acid (GABAergic) projections, playsan
important role in disorders of memory and the treatment of dementias. The GPi
is a major efferent structure of the basal ganglia, using three major
projection systems: the ansa lenticularis, the lenticular fasciculus, and the
pallidotegmental tract. The ansa lenticularis sweeps ventromedially around the
internal capsule, joining the lenticular fasciculus to form the thalamic
fasciculus, which, in turn, projects to different thalamic nuclei, especially
the ventral anterior (VA), ventral lateral (VL), centromedian, and
parafascicular intralaminar nuclei of the thalamus. The pallidotegmental tract
terminates in the pedunculopontine nucleus.
Caudate
Nucleus. The caudate nucleus resembles an elongated and curved exclamation mark. Its
main part is an expanded head directly continuous with a smaller and attenuated
body that merges into an elongated tail. The head bulges into the
anterior horn of the lateral ventricle and forms its sloping floor. The caudate
nucleus is separated from the lentiform nucleus by the anterior limb of the
internal capsule, but the separation is incomplete because the head of the
caudate nucleus and the putamen are connected, especially anteroinferiorly, by
bands of gray matter traversing the white matter of the anterior limb. This
admixture of gray and white matter produces the striated appearance that jus tifies the term “corpus striatum” applied to these
nuclei.
The head tapers
into the narrower body that lies in the floor of the central part of the
lateral ventricle, lateral to the superior surface of the thalamus and
separated from it by a shallow sulcus lodging the stria terminalis and
thalamostriate vein. The tail turns downward along the outer margin of
the posterior surface of the thalamus, with the stria terminalis still lying in
a slight groove between them. It then curves forward into the roof of the
inferior horn of the lateral ventricle, where it separates from the thalamus
and lentiform nucleus by the inferior part of the internal capsule and by
fibers (including some from the anterior commissure) that spread into the
temporal lobe.
Amygdaloid
Body. The
tail of the caudate nucleus ends in a small, almondshaped expansion, the amygdaloid body, which is a complex
of several small nuclei located in the forepart of the roof of the inferior
horn of the lateral ventricle. The stria terminalis issues from the
amygdaloid body and runs along the medial side of the caudate nucleus until it
reaches the vicinity of the ipsilateral interventricular foramen. Here, some of
its fibers join the anterior commissure, others pass to the “septal” region
adjacent to the lamina terminalis, and the remainder descends to the
hypothalamus and anterior perforated substance.
A nuclear
midbrain complex, the substantia nigra (SN), is divided into a pigmented
and dopaminecontaining pars compacta
(SNc) and a cellpoor, pigmentfree pars reticularis (SNr). Most dopaminergic
projections go to the striatum, while a smaller proportion of SNc axons
terminate in the prefrontal cortex. The SNr is a major primary efferent
structure of the basal ganglia, along with GPi. SNr goes primarily to thalamus,
PPN, and the superior colliculus.
A biconvex
structure, the subthalamic nuclei (STN) receives glutamatergic inputs
from the cerebral cortex, GABA inhibition from the GPe, and provides glutama tergic innervations to the GPe, GPi, SN, and PPN. The
STN has become a structure of interest because of its pivotal role in our
understanding of basal ganglia function.
The
postsynaptic dopamine receptors are divided into two major broad categories,
D1/D5 and D2, D3, D4 family of receptors, segregated into two main path ways. The direct pathway, subserved by D1
dopamine receptors, sends its projections to the subthalamic nuclei via the
GPi, and the indirect pathway, via the D2 family of receptors,
influences the STN via the GPe.
Recently, the
excitatoryinhibitory interplay
between the direct and indirect pathways has been conceptual ized as focused selection and tonic inhibition (surround
inhibition hypothesis). By suppressing excitability in an area that is
surrounding an activated neural network, neuronal activity focuses to select
desired responses. Simultaneously, other pallidal neurons projecting to the
thalamus, act to permit desired movements. By decreasing their
discharge, through focused striatal output chiefly via the direct pathway,
tonic inhibition to the thalamus is removed, releasing the cortical generators
for normal or desired movement to occur. Therefore the presence of abnormal
involuntary movements results from either failure of inhibition or excessive
excitation of the surrounding structures.
SCHEMATIC AND CROSS SECTION OF BASAL GANGLIA
Based on the models discussed above, it is important to recognize the pallidum as the major outflow structure of the basal ganglia. Most fugal pathways pass throught the fields of Forel. Presently, the STN is the preferred target for the surgical treatment of idiopathic Parkinson disease (iPD), the ventral intermediate (VIM) thalamus for the treatment of essential and certain other types of tremor, and the GPi for dystonia, with deep brain sti ulation (DBS) being the favored surgical procedure.